What is molecule has a triognal planar shape?
Trigonal planar structure, or trigonal planar shape, are other terms used to express the concept of trigonal planar molecular geometry. Based on electron geometry a molecule, compound, or ion is described as trigonal planar if it has three electron groups. These will all be in the sp^2 hybrid type of orbital.
What determines the molecular geometry?
To predict the shape of a covalent molecule, follow these steps:
- Draw the molecule using a Lewis diagram. Make sure that you draw all the valence electrons around the molecule's central atom.
- Count the number of electron pairs around the central atom.
- Determine the basic geometry of the molecule using the table below. ...
What is planar in chemistry?
What does planar mean in organic chemistry? The technical term “planar” refers to the arrangement of bonds around the central atom in covalent molecules. If the atoms are bonded to the central atom in the same face it is called a planar arrangement - “in one plane”.
Which molecule is linear in shape?
Which molecule has a linear geometry? Linear molecule is a molecule in which atoms are deployed in a straight line (under 180° angle). Molecules with an linear electron pair geometries have sp hybridization at the central atom. An example of linear electron pair and molecular geometry are carbon dioxide (O=C=O) and beryllium hydride BeH2.
Is BF3 trigonal planar?
BF3 Molecular Geometry and Bond Angles To be more precise, the BF3 molecular geometry is trigonal planar. It further has symmetric charge distribution on the central atom and is nonpolar. The bond angle is 120o where all the atoms are in one plane. Each of them also makes an equilateral triangle.
Is NF3 trigonal planar?
The molecule NF3 N F 3 does not have a trigonal planar structure. The correct molecular geometry for the molecule is trigonal pyramidal. It has a trigonal pyramidal structure because the Nitrogen atom (central atom) is bonded to 3 F atoms and contains a lone pair.
What molecules have a trigonal shape?
Phosphine, an example of a molecule with a trigonal pyramidal geometry.
What shape is sf4?
Sulfur tetrafluorideNamesStructureMolecular shapeSeesaw (C2v)Dipole moment0.632 DHazards40 more rows
What is the shape of nh3?
trigonal pyramidal shapeThe ammonia molecule has a trigonal pyramidal shape with the three hydrogen atoms and an unshared pair of electrons attached to the nitrogen atom.
Is NH3 trigonal planar?
With three hydrogen atoms and an unshared pair of electrons connected to the nitrogen atom, NH3 molecular geometry is of Trigonal Pyramidal shape.
Which has triangular planar shape?
CH3+ has trigonal planar shape. There are 3 pairs of electrons around the carbon atom and C− atom in CH3+ possess sp2-hybridization.
Is carbon dioxide trigonal planar?
Molecular Geometry And Vsepr : Example Question #2 The nitrogen atom in ammonia has three hydrogens attached, as well as a lone pair, in order to satisfy its octet. This gives ammonia a trigonal pyramidal geometry. Methane has a tetrahedral geometry, carbon dioxide is linear, and boron trifluoride is trigonal planar.
Is NF3 trigonal pyramidal?
The molecular geometry or shape of NF3 is a trigonal pyramid and its electron geometry is tetrahedral. NF3 lewis dot structure contains 1 lone pair and 3 bonded pairs.
What is the shape of NF3?
trigonal pyramidalThe molecule has a trigonal pyramidal structure, like ammonia.
Why is NF3 pyramidal?
Its three electrons are used to form bond with three F - atoms, so one lone pair of electrons is present in this molecule on N-atom. It shows lp -bp repulsion which is more than bp-bp repulsion. So, its shape is pyramidal. While in BF3, B is surround by three F- atoms.
What kind of bond is NF3?
covalent compoundsThe molecule BF3 and NF3 , both are covalent compounds but BF3 is non - polar and NF3 is polar.
What molecules are trigonal planar?
The molecules of BF 3 , BCl 3 , AlCl 3 , SO 3 , AlF 3 etc show trigonal planar geometry. These molecules show sp 2 hybridisation and bond angle 1...
What angle is trigonal planar?
A trigonal planar molecule has a central atom bonded to three surrounding atoms, with no lone electron pairs. Therefore, its steric number is three...
What is the difference between trigonal planar and tetrahedral?
A trigonal planar molecule has a central atom bonded to three surrounding atoms, with no lone electron pairs. Therefore, its bond angle is 120 0 ....
Is sp2 trigonal planar?
The geometry of the sp 2 hybrid orbitals is trigonal planar , with the lobes of the orbitals pointing towards the corners of a triangle. The angl...
How many lone pairs are in trigonal planar?
There are zero lone pairs present in the trigonal planar geometry.
What is a trigonometric planar molecule?
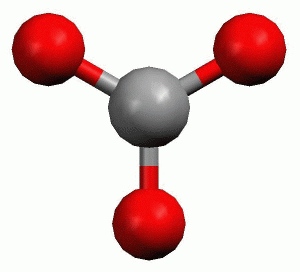
Structure of boron trifluoride, an example of a molecule with trigonal planar geometry. In chemistry, trigonal planar is a molecular ge ometry model with one atom at the center and three atoms at the corners of an equilateral triangle, called peripheral atoms, all in one plane.
What is the bond angle of a trigonal planar species?
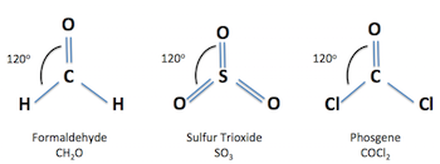
In an ideal trigonal planar species, all three ligands are identical and all bond angles are 120°. Such species belong to the point group D 3h. Molecules where the three ligands are not identical, such as H 2 CO, deviate from this idealized geometry. Examples of molecules with trigonal planar geometry include boron trifluoride (BF 3 ), ...
Is boron trifluoride a trigonal planar molecule?
Structure of boron trifluoride, an example of a molecule with trigonal planar geometry. In chemistry, trigonal planar is a molecular geometry model with one atom at the center and three atoms at the corners of an equilateral triangle, called peripheral atoms, all in one plane. In an ideal trigonal planar species, ...
What is the molecule's shape with F and H atoms?
SHAPE? Tetrahedral, with F atoms at two points, and H atoms at the other two. This gives the molecule a hydrogen"side" and a fluorine "side" ... making it POLAR.
How are sigma bonds formed?
SIGMA bonds are formed when orbitals overlap along the axis between two atoms. Thesebonds have good overlap between the bonding orbitals, meaning that they are strong. Single bonds are always sigma bonds. Double and triple bonds contain onesigma bond each.
How many atoms are bonded to the central sulfur atom?
There are several derivatives of the trigonal bipyramidal shape (like the tetrahedral shape) - depending on how many things around the central atom are atoms!There are six atoms bonded to the central sulfur atom, and they will attempt to get as far apart as possible from one another!
What Is Molecular Geometry?
- The arrangement of atoms in a molecule, which is usually relative to a single central atom, is referred to as molecular geometry. It excludes lone pairs from determining a molecule’s shape, though repulsion from lone pair(s) is taken into account only in bond angles. Bond lengths, bon…
What Is Trigonal Planar Molecular Geometry?
- A trigonal planar molecular geometry model has one atom in the centre and three atoms at the corners of an equilateral triangle, known as peripheral atoms, all in the same plane. All three ligands in an ideal trigonal planar species are identical, and all bond angles are 120°. Molecules with three ligands which are non-identical deviate from this idealised geometry.
Table of Contents
Trigonal Planar Molecular Geometry Bond Angle
- The geometry described by trigonal planar molecular geometry revolves around a central atom that is bonded at a bond angle of 120° to three other atoms (or ligands).
Trigonal Planar Molecular Geometry Lone Pairs
- The trigonal planar molecular geometry has 0 lone pairs. When there are 1 lone pair and 2 bond pairs present, the molecular geometry becomes bent or angular, whereas the electron geometry remains the same – trigonal planar. Read more:Molecular Geometry and Electron Geometry
Trigonal Planar Molecular Geometry Polarity
- A trigonal planar molecule is nonpolar because the bond polarities cancel each other out, it is also due to the perfect symmetry. A molecule cannot be polar if all three positions are the same, a bent or angular molecule is polar.
Trigonal Planar Molecular Geometry Hybridisation
- The sp2hybridisation is often used to describe planar, three-connected carbon centres that are trigonal planar. When all of the bonds are in place, the shape is trigonal planar, and the shape bends if there are only two bonds and one lone pair of electrons holding the place where a bond would be.