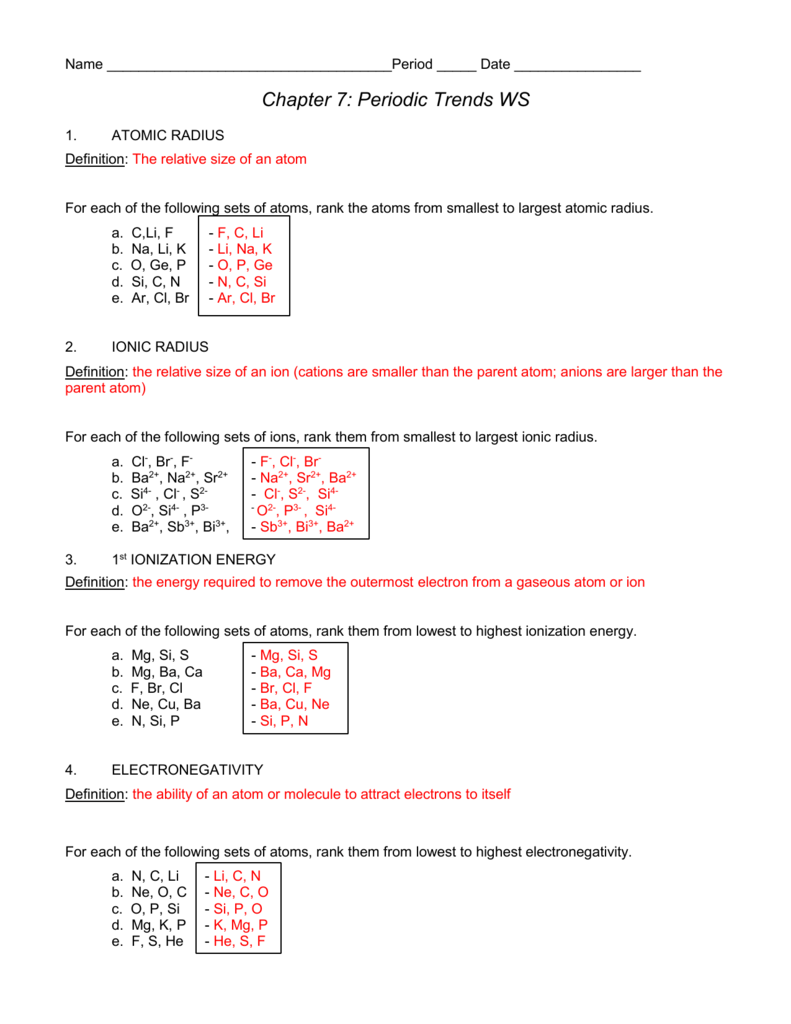
What period are the following elements in GE?
| Group | 14 | 938.25°C, 1720.85°F, 1211.4 K |
| Period | 4 | 2833°C, 5131°F, 3106 K |
| Block | p | 5.3234 |
| Atomic number | 32 | 72.630 |
| State at 20°C | Solid | 73Ge, 74Ge |
How to see the period of all the elements of periodic table?
Mouseover on the chart to see the element name and Period of the element. This Period chart table gives the Period of all the elements of periodic table . Click on 'Element Atomic Number', 'Element Symbol', 'Element Name' and 'Element Period' headers to sort. Loading, please wait... In the below periodic table you can see the trend of Period.
What are the new elements added to the 7th period?
The 7th period of the periodic table now has four new elements: element 113 (temporarily named as Ununtrium, or Uut), element 115 (Ununpentium, or Uup), element 117 (Ununseptium, or Uus), and element 118 (Ununoctium, or Uuo), says a group of experts from the International Union of Pure and Applied Chemistry (IUPAC) and
What are the characteristics of a group on the periodic table?
A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. A horizontal row in the periodic table.
What determines the phase change of a noble gas?
The arrangements of electrons above the last (closed shell) noble gas. The temperature at which the solid–liquid phase change occurs. The temperature at which the liquid–gas phase change occurs.

What period element is GE?
period 4GermaniumAtomic number (Z)32Groupgroup 14 (carbon group)Periodperiod 4Blockp-block49 more rows
What group are the following elements in GE?
Group 4A (or IVA) of the periodic table includes the nonmetal carbon (C), the metalloids silicon (Si) and germanium (Ge), the metals tin (Sn) and lead (Pb), and the yet-unnamed artificially-produced element ununquadium (Uuq).
What period are the following elements in a he?
Period 1Fact boxGroup18Melting pointPeriod1Boiling pointBlocksDensity (g cm−3)Atomic number2Relative atomic massState at 20°CGasKey isotopes2 more rows
What period are the following elements in RB?
Period 5Fact boxGroup139.30°C, 102.74°F, 312.45 KPeriod5688°C, 1270°F, 961 KBlocks1.53Atomic number3785.468State at 20°CSolid85Rb, 87Rb2 more rows
What element is in Group 4 Period 5?
ZirconiumThe symbol of the element in Group 4 and Period 5 is Zr. It is the symbol of Zirconium.
What element is in group 16 Period 6?
This group is also known as the oxygen family. It consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive element polonium (Po)....Atomic and physical.ZElementNo. of electrons/shell8Oxygen2, 616Sulfur2, 8, 634Selenium2, 8, 18, 652Tellurium2, 8, 18, 18, 62 more rows
What is period 5 on the periodic table?
The period 5 transition metals are yttrium (Y), zirconium (Zr), niobium (Nb), molybdenum (Mo), technetium (Tc), ruthenium (Ru), rhodium (Rh), palladium (Pd), silver (Ag), and cadmium (Cd).
What element is in Period 3?
The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block.
What element is in period 4 Group 7?
ManganeseThe element the is present in period 4 Group 7 in the periodic table is Manganese. It is represented by the symbol Mn and has an atomic number of 25. Manganese belongs to the category of transition metals.
What element has a period 5 in the group of 10?
Group 10, numbered by current IUPAC style, is the group of chemical elements in the periodic table that consists of nickel (Ni), palladium (Pd), platinum (Pt), and perhaps also the chemically uncharacterized darmstadtium (Ds).
What is RB periodic table?
rubidium (Rb), chemical element of Group 1 (Ia) in the periodic table, the alkali metal group. Rubidium is the second most reactive metal and is very soft, with a silvery-white lustre.
Which element is in period 5 Group 2?
"Beryllium." Periodic Table of Elements and Chemistry.