
What are some facts about hydrogen peroxide?
Hydrogen Peroxide
- Uses & Benefits. Hydrogen peroxide is used as an antimicrobial agent and an oxidizing agent. ...
- Safety Information. Hydrogen peroxide-based products purchased by consumers for household use typically contain around 3 percent hydrogen peroxide.
- Answering Questions. What are some additional uses of hydrogen peroxide? ...
What are the health benefits of hydrogen peroxide?
Household uses for hydrogen peroxide
- Cleaner and disinfectant. Hydrogen peroxide is an effective cleaning and disinfecting agent around the house, from cleaning the dishwasher to scrubbing the sink to sanitizing countertops.
- Washing vegetables. ...
- Cleaning cookware. ...
- Stain remover and whitener. ...
- Brush cleaner and sanitizer. ...
Does hydrogen peroxide really bleach hair?
That hydrogen peroxide sitting around in your house in brown bottle is not only effective for treatment of cuts and mildew but can also be used to bleach the hair. It is in fact one of the most commonly used hair bleaching agents. And if you didn’t know, hydrogen peroxide bleached hair is also often referred to as peroxide blonde.
Can hydrogen peroxide be harmful?
This type of hydrogen peroxide can cause minor symptoms to occur if ingested, touched, or inhaled. More potent forms of hydrogen peroxide can be dangerous — or even fatal — to drink, breath in, or touch. Hydrogen peroxide is not a cure for cancer, HIV, or any other disease.
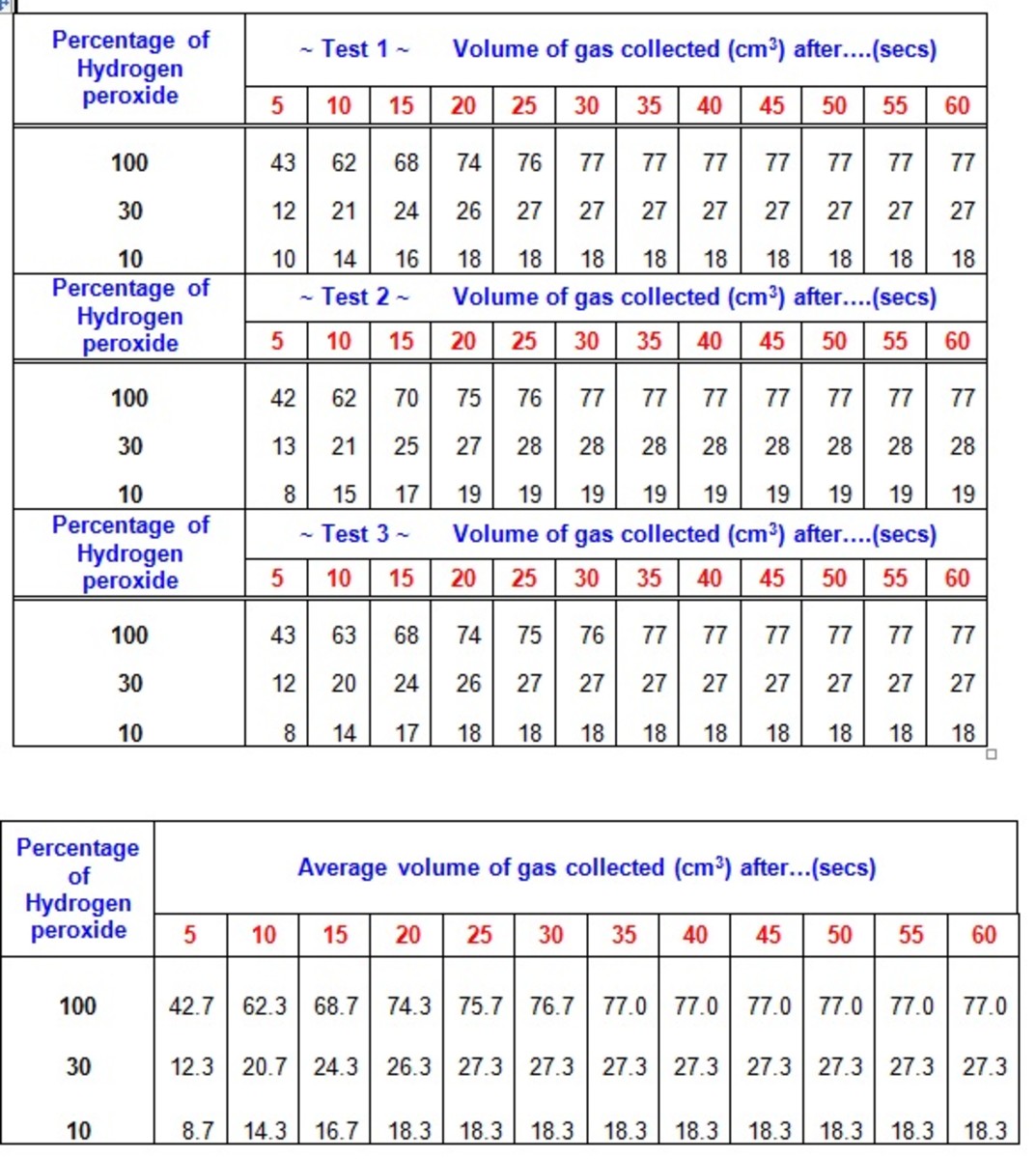
Does pH affect hydrogen peroxide?
The efficacy of hydrogen peroxide bleaching is directly proportional to the increase of its pH. The significant increase in bleaching outcomes occurs from pH 6.0, with maximum effectiveness achieved with pH 9.0.
What pH is optimal for catalase?
Catalase has an optimum pH of 9 and a working range of between pH 7-11. Most other enzymes function within a working pH range of about pH 5-9 with neutral pH 7 being the optimum.
Why is pH 7 optimum for catalase?
Catalase pH Levels If the pH level is lower than 7 or higher than 11, the enzyme becomes denaturated and loses its structure. The liver sustains a neutral pH of about 7, which creates the best environment for catalase and other enzymes.
At what pH is enzyme activity the highest?
around 5.0Maximal activity was observed for pH-values around 5.0 for chitosanase from T. harzianum and T. viride for any considered temperature. For these strains, the maximal enzyme activity was obtained around pH 5.0 and higher or lower pH-values strongly decreased the chitosanase activity.
How does pH affect the reaction rate?
Optimal pH increases enzyme rate of reaction while less than optimal pH decreases it. Increasing temperature also increases enzyme rate of reaction, until things get too hot, then the enzyme denatures and ceases to function.
Does catalase work better in acid or base?
The catalase enzyme works best around pH 7, which is pH neutral. This indicates that it works best when there is neither excess acid nor base.
Why does high pH affect enzyme activity?
Changing the pH will affect the charges on the amino acid molecules. Amino acids that attracted each other may no longer be. Again, the shape of the enzyme, along with its active site, will change. Extremes of pH also denature enzymes.
What is an optimal pH?
The optimum pH for our blood and body tissues is about 7.2. (The use of saliva and urine test strips will show a much lower pH level due to the protein present in the solution. Saliva and urine tests from a healthy body should be about 6.6 to 6.8.)
What is pH value of pure water or human blood?
A normal blood pH level is 7.35 to 7.45 on a scale of 0 to 14, where 0 is the most acidic and 14 is the most basic. This value can vary slightly in either direction.
At what pH is the rate of reaction the highest lowest explain?
Activity is usually highest at pH 10 and lowest at pH 4.
What is the optimum pH of the catalase quizlet?
- Catalase works best at pH 7.
What is the optimal pH for catalase Why do you think it becomes less effective at high or low pH?
In the case of catalase, the optimum pH is approximately pH 7.0. That is, catalase works best at a neutral pH. If the solution is too acidic (low pH value) or too basic (high pH value) the catalase is inactive and no longer functions as an enzyme.
What is the chemical formula for hydrogen peroxide?
To begin with, hydrogen peroxide is a compound that looks like a very light-blue and clear liquid. Its chemical formula is H2O2. It is a bit more viscous compared to water and this compound is very unstable.
Why is peroxide mixed with bleach?
Peroxide must bever be mixed with bleach because of the threat of an explosion. Also, vinegar and H2O2 create a highly corrosive substance that is dangerous to our skin, eyes, and lungs.
What Is PKA In Chemistry?
Except for the pH rate, we can often hear about the pKa of these or other solutions or compounds. Hydrogen peroxide acid is not an exception.
What does pKa mean in acid?
To be short, the pKa value indicates how strong an acid is. The lower the value the stronger the acid and vice versa. Respectively, stronger acids separate in water better. Speaking of peroxide acid, it is good to know to be able to calculate how much of it one will need for certain purposes, for instance, or how it will react with water. ...
What is the pH scale?
The pH scale ranges from zero to fourteen where the indicators from zero to six refer to acids, seven is for neutral solutions and the indicators from eight to fourteen belong to basic compounds.
Is hydrogen peroxide a solid?
Yes, there are several compounds that contain hydrogen peroxide and are solid. One is urea peroxide and another is sodium percarbonate. When dissolved, they both give a high concentration of H2O2.
Is hydrogen peroxide a household substance?
Since hydrogen peroxide is widely used in a household, it will be useful to know the answers to several most widespread questions regarding this substance. Like that, you’ll be able to work with this compound correctly.
What is the pH of hydrogen peroxide?
A solution which only contains hydrogen peroxide and water can have pH values between approximately 4.3 and 7.0 at 25 °C. The lowest pH value is measured at a percent concentration of roughly wt%=50. 7.0 is the upper limit at infinite dilution.
How to force hydrogen peroxide to act as a base?
You could try to force hydrogen peroxide to act as a base by putting it in an acid solution, but acids catalyze H2O2's breakdown rapidly to water + oxygen (start with the equation above, then allow the HO+ to dissociate to H+ + O radical; 2 moles give you 2 of water via the H+ and OH-, and 1 mole of O2), so good luck.
How does hydrogen peroxide decompose?
It will decompose over time to water and oxygen. It oxidizes things by releasing an oxygen atom as a free radical (really toothy mean form of oxygen). If you want to break hydrogen peroxide, just sneeze. There is a plant called a touch-me-not that grows in ditches.
Why does H2O2 hurt?
Ever wonder why putting H2O2 on an untreated cut feels like putting your hand on top of a stove? That’s because the compound is killing harmful bacteria (and other healthy cells) in the form of a reaction . Living cells in your body have an enzyme called catalase that functions as a catalyst when decomposing hydrogen peroxide. Upon contact, the catalase will catalyze with H2O2, immediately converting it into water and oxygen gas. In a nutshell, the stinging sensation comes from the H2O2 harming exposed tissues by breaking down cell membranes and disrupting cell functionality, and the pain will linger if not washed off immediately.
Why does H2O2 sting?
Ever wonder why putting H2O2 on an untreated cut feels like putting your hand on top of a stove? That’s because the compound is killing harmful bacteria (and other healthy cells) in the form of a reaction . Living cells in your body have an enzyme called catalase that functions as a catalyst when decomposing hydrogen peroxide. Upon contact, the catalase will catalyze with H2O2, immediately converting it into water and oxygen gas. In a nutshell, the stinging sensation comes from the H2O2 harming exposed tissues by breaking down cell membranes and disrupting cell functionality, and the pain will
What happens if you dilute H2O2?
If you dilute the H2O2 by 50%, the burning sensation will likely cease.
What is 80%-90% used for?
The commonest use for it at this concentration is industrial, for subsequent dilution to lower concentrations for use in the food and medical industries for example, however 80%-90% concentrations are used as a propellant in small jet engines, as used in say personal jet packs and for vertical take-off autogyros, where small H2O2 jet engines are fixed to the rotor tips.
What happens when the pH of hydrogen peroxide is high?
However, when the pH is high, there is the formation of insoluble iron hydroxide (colloidal hydroxide), which exerts higher catalytic activity for the peroxide decomposition than the complex peroxide or per-ions. With a further increase of pH and excess of alkalinity, these hydroxides are redissolved and release the metal ions, which increase the catalytic activity even more, therefore increasing the bleaching effect. 39
What is hydrogen peroxide used for?
When in contact with oral tissues, the molecule is decomposed, producing highly reactive free radicals. For dental bleaching procedures , two main techniques are available. The at-home bleaching technique uses hydrogen peroxide gel in low concentrations, up to 10%, in trays or strips applied over the patient's teeth with no special isolation, without major irritative effects on the soft tissues. However, for the in-office technique, highly concentrated hydrogen peroxide gels, up to 38%, are applied over the enamel but with some kind of previous gingival isolation, preventing the gel from contacting the soft tissues. If this contact occurs, a chemical burning is always expected by the oxidative effect of hydrogen peroxide, even in a neutral pH. According to the OECD Guidelines for Testing of Chemicals, related to acute dermal irritation/corrosion, substances exhibiting pH extremes such as <2.0 and >11.5 may have strong local effects, identifying those substances as corrosives to skin or mucosa. 42 If the pH is >2.0 and <11.5, it is assumed as not corrosive or irritative in relation to pH. In our study, the pH range tested was 3-9. Therefore, no irritative effects are expected for a bleaching compound in this range, with the possible aggressive effects related to the oxidative action of hydrogen peroxide by itself.
What happens when you increase pH?
The latter leads to the formation of free radicals ( equation 2 ), which are the active species of oxidizing chromogens. Stoichiometric experiments showed that the formation of perhydroxyl ion is influenced by pH; thus, the higher the pH, the more ions are formed, leading to more free radical production. The perhydroxyl anion is the primary key species responsible for the bleaching outcomes. In one experiment with cotton fabric, the degree of bleaching obtained was directly related to the increase in pH and the concentration of perhydroxyl anion in the whitening solution. 35 An increase of oxygen release as a result of the increased pH has also been observed. 36 - 38
Why are bleaching solutions acidic?
Many bleaching solutions and gels manufactured in a single bottle, ready for use, are acidic or neutral, in order to make the chemical stabilization of hydrogen peroxide easier, preventing their decomposition. This aims to prevent the formation of oxygen and water inside the bottle, thus enhancing the validity of the product, although its whitening efficacy is suppressed. 8, 9, 40, 41 The high level of hydrogen peroxide stability and its reduced catalytic activity in acid systems were attributed to the lack of an initiator necessary to decompose hydrogen peroxide into free radicals. 41 The perhydroxyl ion, which is supposed to start the decomposition of hydrogen peroxide in alkaline solutions, is present in insignificant amounts in acidic solutions since the ionization of hydrogen peroxide is not favored.
What is the most commonly used bleaching agent?
Hydrogen peroxide is the most widely used dental bleaching agent, manufactured at low concentration for at-home techniques or high concentration for in-office techniques. 4 - 6 It is a highly unstable molecule and decomposes according to a sequence of reactions, which can be influenced by incident light, pH, temperature, interactions with transition metals, and other factors. 7 - 9 Initially, it decomposes into hydrogen cations (H +) and the perhydroxyl anion (HO 2−; equation 1 ). The H + release explains their behavior as a weak acid. The perhydroxyl anion interacts with another peroxide molecule and results in the formation of the free radicals hydroxyl (HO)• and perhydroxyl (HO 2 )•, also called active species ( equation 2 ). The hydroxyl radicals react with more peroxide and result in the formation of more perhydroxyl radicals and water ( equation 3 ). With the completion of the reaction, all of the peroxide is converted to water. Obviously, the description above is simplified due to the possible formation of intermediates and other active species.
Why is hydrogen peroxide used in bleaching?
Hydrogen peroxide solutions with a higher pH are used to increase the efficacy of the process. 12 In relation to dental bleaching gels, a large number of products present an acidic pH in order to increase the product's shelf life, since hydrogen peroxide is more stable in an acidic environment.
What factors affect the decomposition of hydrogen peroxide?
The factors that change the decomposition of hydrogen peroxide include impurities, temperature, pH, and metal ions from solutions. 8, 10, 32 As it concerns pH, the results reported in our study, directly measuring the color of the solution containing the chromogen, clearly show that the efficacy of hydrogen peroxide bleaching is directly proportional to the pH of the solution. The significant increase in bleaching outcomes occurs from pH 6.0, with maximum effectiveness achieved at pH 9.0, both for wine and tobacco solutions; thus, the null hypothesis was rejected.
How much hydrogen peroxide is toxic?
Note: Hydrogen Peroxide (52% by weight or greater) in quantities at or above above 7500lb presents a potential for a catastrophic event as a toxic or reactive highly hazardous chemical.
Where is hydrogen peroxide found?
Atmospheric hydrogen peroxide is believed to be generated exclusively by gas-phase photochemical reactions. It has been found in rain and surface water, in human and plant tissues , in foods and beverages and in bacteria (1). Hydrogen peroxide occurs in cloud water with higher values generally occurring in the vicinity of lightning activity (2).
How many workers are exposed to hydrogen peroxide?
According to the 2016 TSCA Inventory Update Reporting data, 20 reporting facilities estimate the number of persons reasonably likely to be exposed during the manufacturing, processing, or use of hydrogen peroxide in the United States may be as low as 10 workers to less than 10,000 but unknown or unreasonably ascertainable as to how many workers per plant; the data may be greatly underestimated due to confidential business information (CBI) or unknown values (1).
How explosive is hydrogen peroxide?
Although pure hydrogen peroxide solutions are not usually explosive at atmospheric pressure, equilibrium vapor concentrations of hydrogen peroxide above 26 mol per cent (40 weight per cent) become explosive in a temperature range below the boiling point of the liquid.
What enzyme reduces hydrogen peroxide?
Hydrogen peroxide is reduced by glutathione peroxidase, which is an endogenous enzyme in human tissue. It is rapidly decomposed to oxygen and water when in contact with catalase, an enzyme found in blood and most tissues [L2024].
How do hydroquinones react with hydrogen?
After the catalyst is removed (otherwise, the hydrogen peroxide would decompose), the hydroquinones are oxidized to quinones with oxygen (usually air) with simultaneous quantitative formation of hydrogen peroxide. Hydrogen peroxide is extracted with water, and the quinones are returned to the hydrogenator to complete the loop. The AO process, therefore, leads to the net formation of hydrogen peroxide from gaseous hydrogen and oxygen.
Is hydrogen peroxide safe for humans?
The US Food and Drug Administration (FDA) has alerted consumers that high strength hydrogen peroxide preparations (e.g., 35% food grade hydrogen peroxide) should not be used for any medicinal purpose. These preparations are being promoted on websites illegally for various medicinal purposes (e.g., AIDS, cancer, emphysema, other life-threatening conditions) without any proven clinical value, and such uses are dangerous. Hydrogen peroxide 35% is not approved by the FDA for any purpose. Ingestion of such preparations can cause serious harm or death. Oral ingestion of hydrogen peroxide can result in GI irritation and ulceration. IV administration of hydrogen peroxide can result in inflammation at the injection site, gas embolism, and life-threatening allergic reactions.
What is hydrogen peroxide used for?
Hydrogen peroxide, a chemical that appears as a colorless liquid, is used in a wide range of cleaning and personal care products, including hair dyes and bleaches, toothpaste and mouthwashes, bathroom cleaners and laundry stain removers. Hydrogen peroxide can also be found in over-the-counter (OTC) first aid antiseptics, ...
What is the maximum amount of peroxide per million?
The National Institute for Occupational Safety and Health’s permissible exposure limits for hydrogen peroxide at higher concentrations is 1 part per million over an 8-hour work shift.
How much hydrogen peroxide is in toothpaste?
Hydrogen peroxide-based products purchased by consumers for household use typically contain around 3 percent hydrogen peroxide. Manufacturer’s instructions should be followed for safe use. The European Commission on Health & Consumer Protection conducted extensive testing on the safety of hydrogen peroxide in teeth whitening products ...
Is hydrogen peroxide an antiseptic?
Hydrogen peroxide is used as an antimicrobial agent and an oxidizing agent. It is also used as an OTC antiseptic to clean wounds. In personal care products, such as colorants and toothpastes, hydrogen peroxide works as an oxidizing agent, offering a lightening/whitening effect.
Is hydrogen peroxide safe for wounds?
FDA also has stated that hydrogen peroxide is “ safe for use as an oral wound healing agent .”
Can hydrogen peroxide be used as a mouth rinse?
In low concentrations, hydrogen peroxide can be used as a mouth rinse to remove mucus or minor mouth irritations. In the home, it can also be used to help remove mold and mildew stains from dishwashers, disinfect counters and cutting boards, and wash vegetables by removing bacteria from them.
What is Hydrogen Peroxide?
Hydrogen peroxide is a compound with the chemical formula H2O2. It has a variety of uses as both a household cleaning product and as a natural remedy.
What to wear when using hydrogen peroxide?
When using hydrogen peroxide, always wear protective clothing such as goggles and gloves to avoid any contact.
How to make a paste with hydrogen peroxide?
To make a paste, mix a few drops of hydrogen peroxide with 2-3 teaspoons of baking soda. Mix it well till you get a smooth paste.
What happens when you put hydrogen peroxide on a cut?
When you use hydrogen peroxide on a cut, it comes in contact with your damaged cells and blood. It also comes in contact with catalase, an enzyme present in our body that further breaks it down and turns H2O2 into O2 & H2O. The bubbles are oxygen gas making their way out.
How is hydrogen peroxide formed?
Hydrogen peroxide is a simple chemical compound known for its antiseptic properties. It is formed when water molecules bond with oxygen atoms from hydrogen molecules.
How to use hydrogen peroxide in ear drops?
How to use these ear drops containing hydrogen peroxide? Before you start, lay down on your side to ensure that one ear is up. Then, put the drops in your ears and let the liquid stay in your ears for about 5 minutes. Next, sit and take a tissue to clean any extra liquid that comes out of your ears. Once done, do this with the other ear. This should do the job!
Can you use hydrogen peroxide to remove mold?
Fortunately, it is possible to remove mold with hydrogen peroxide.
