- Household Items. Sulfuric acid is in numerous household cleaners, particularly toilet bowl cleaners and drain de-cloggers.
- Dangers. Sulfuric acid in household products can be dangerous in two distinct ways. As a corrosive acid, it can burn through human skin and many household surfaces and materials.
- Safe Handling. The most important and most effective step for the safe handling of a product containing sulfuric acid is to follow the product's directions and safety warning meticulously.
- Other Considerations. When you cut an onion, the onion's cell walls break open and release propanethiol S-oxide. ...
What are the common uses of sulfuric acid?
- For the manufacture of fertilizers, ammonium sulphate, and superphosphate.
- In the manufacture of other important industrial chemicals such as hydrochloric acid, nitric acid, sulphates of metals, alums, sodium carbonate, etc.
- In the preparation of dyes, drugs, and disinfectants.
What are the uses of sulphuric acid?
Up to 763,000 gallons of sulfuric acid waste coming from Austin’s Samsung facility spilled ... 2022 American City Business Journals. All rights reserved. Use of and/or registration on any portion of this site constitutes acceptance of our User Agreement ...
What are the hazards of sulfuric acid?
Sulfuric acid
- 1 Structures
- 2 Names and Identifiers. ...
- 3 Chemical and Physical Properties. ...
- 4 Spectral Information. ...
- 6 Chemical Vendors
- 7 Drug and Medication Information
- 8 Food Additives and Ingredients. ...
- 9 Agrochemical Information. ...
- 10 Pharmacology and Biochemistry
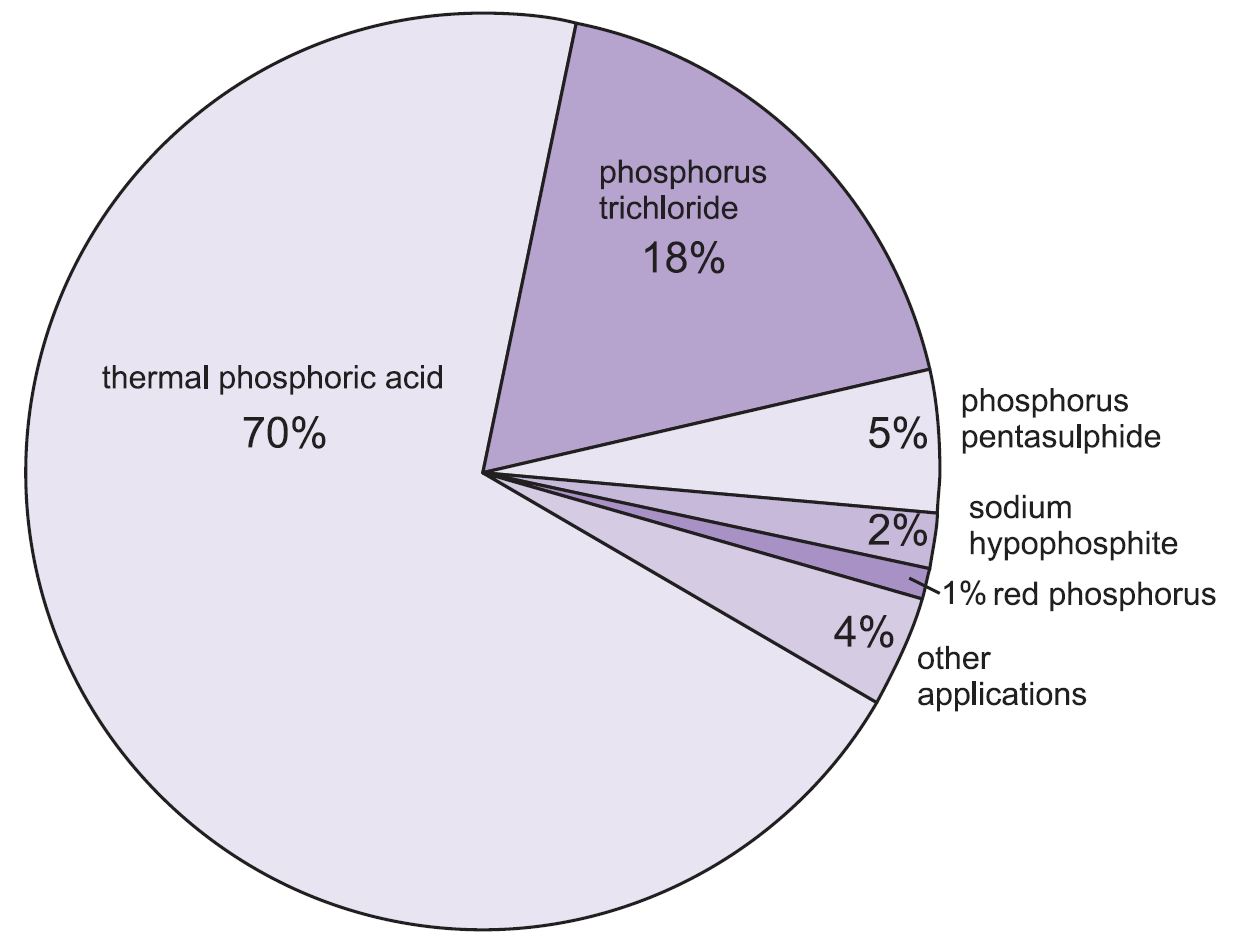
- 11 Use and Manufacturing. ...
What are the agricultural uses of sulfuric acid?
Uses of Sulphuric Acid
- Uses of Sulphuric Acid at Home. It is a very dangerous chemical and should be handled carefully. ...
- Industrial Uses of Sulfuric Acid
- Uses of Sulfuric Acid in the Production of Drugs. Used to damage the cancerous cell DNA by the manufacture of chemotherapy drugs. ...
- Sulphuric Acid on Skin. It is used in skin ointments to treat skin infections like canker sores. ...

What household items have Sulphuric acid?
One of the most common examples of sulfuric acid in the home is drain cleaner; liquid-form drain cleaners that unclog drains often contain sulfuric acid. In lesser concentrations, sulfuric acid occurs in glass-cleaning etching compounds, rust and corrosion dissolvers and some fabric cleaners.
Where is sulfuric acid commonly found?
Sulfuric acid is a constituent of acid rain, being formed by atmospheric oxidation of sulfur dioxide in the presence of water - i.e. oxidation of sulfurous acid. Sulfur dioxide is the main product when the sulphur in sulfur-containing fuels such as coal or oil is burned.
What is sulfuric acid used for at home?
Cleaning and Dyestuffs Sulfuric acid is used to manufacture cleaning products, such as detergents and bleaches. It is used in many cleaning products, such as oven cleaners and toilet bowl cleaners. It can also make dyestuffs for clothes because it is a very good solvent.
How do you make sulfuric acid at home?
0:004:25How to make sulfuric acid from sulfur - YouTubeYouTubeStart of suggested clipEnd of suggested clipHello and welcome to the science fairy in this video i will show you how to make sulfuric acid.MoreHello and welcome to the science fairy in this video i will show you how to make sulfuric acid. First we need the most important ingredient. And that is sulfur i directly ordered one kilogram.
How do we get sulfuric acid?
How Sulphuric acid is produced? Sulphuric acid is produced from sulphur. In the presence of air, sulphur dioxide is first obtained by burning the molten sulphur. In the presence of a catalyst for vanadium pentoxide, sulphur dioxide is then converted to sulphur trioxide.
What cleaners are sulfuric acid?
In the United States, you can buy sulfuric acid drain cleaner in big box stores under brand names such as Kleen-Out, Clean Shot and Liquid Lightning. These are 93 to 95 percent sulfuric acid solutions, which means they're highly concentrated, so you must treat them with respect.
Is sulfuric acid used in cosmetics?
Sulfuric acid esters are used as surfactants in cosmetics, like sodium laureth sulfate.
Is sulfuric acid in batteries?
Battery acid is sulfuric acid that has been diluted with water to attain a 37% concentration level. This particular type of acid is used in sealed lead acid batteries, however, concentration levels differenciate with some brands.
Is sulfuric acid naturally occurring?
Pure sulfuric acid is not encountered naturally on Earth in anhydrous form, due to its great affinity for water. Dilute sulfuric acid is a constituent of acid rain, which is formed by atmospheric oxidation of sulfur dioxide in the presence of water – i.e. oxidation of sulfurous acid.
What is the strongest acid in the world?
Fluoroantimonic acidFluoroantimonic acid is the strongest super-acid known in existence which is 100,000 billion billion billion times more acid than gastric acid (pH of -31.3.). This substance is so strong it will eat through skin, bones, and pretty much any container used to store it.
What happens if you drink sulfuric acid?
Drinking concentrated sulfuric acid can burn your mouth and throat, and it can erode a hole in your stomach; it has also resulted in death. If you touch sulfuric acid, it will burn your skin. If you get sulfuric acid in your eyes, it will burn your eyes and cause them to water.
Does sulfuric acid burn skin?
Exposure to sulfuric acid may occur through skin contact, eye contact, ingestion, and breathing contaminated air. Sulfuric acid can cause severe skin burns, it can burn the eyes, burn holes in the stomach if swallowed, irritate the nose and throat, and cause difficulties breathing if inhaled.
What is sulfuric acid used for?
Sulfuric acid is also a key substance in the chemical industry. It is most commonly used in fertilizer manufacture, but is also important in mineral processing, oil refining, wastewater processing, and chemical synthesis.
What is the chemical formula for sulfuric acid?
Sulfuric acid ( American spelling) or sulphuric acid ( Commonwealth spelling ), also known as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula H 2 SO 4. It is a colorless, odorless and viscous liquid that is miscible with water.
What is the grade of sulfuric acid?
Grades of sulfuric acid. Although nearly 100% sulfuric acid solutions can be made, the subsequent loss of SO. 3 at the boiling point brings the concentration to 98.3% acid. The 98.3% grade is more stable in storage, and is the usual form of what is described as "concentrated sulfuric acid".
How is sulfur dioxide oxidized?
The sulfur dioxide is oxidized to sulfur trioxide by oxygen in the presence of a vanadium (V) oxide catalyst. This reaction is reversible and the formation of the sulfur trioxide is exothermic. 7 ), also known as fuming sulfuric acid. The oleum is then diluted with water to form concentrated sulfuric acid.
What is the concentration of sulfuric acid?
Although nearly 100% sulfuric acid solutions can be made, the subsequent loss of SO#N#3 at the boiling point brings the concentration to 98.3% acid. The 98.3% grade is more stable in storage, and is the usual form of what is described as "concentrated sulfuric acid". Other concentrations are used for different purposes. Some common concentrations are:
How does sulfuric acid decompose?
Drops of concentrated sulfuric acid rapid ly decompose a piece of cotton towel by dehydration. Play media. An experiment that demonstrates the dehydration properties of concentrated sulfuric acid. When concentrated sulfuric acid comes into contact with sucrose, slow carbonification of the sucrose takes place.
Why does acid not boil?
Acid will not boil, because of its higher boiling point. Warm water near the interface rises due to convection, which cools the interface, and prevents boiling of either acid or water. In contrast, addition of water to concentrated sulfuric acid results in a thin layer of water on top of the acid.
What are the sources of sulfur?
Major sulfur-producing sources include sedimentary rocks, which release hydrogen sulfide gas, and human sources, such as smelters and fossil-fuel combustion, both of which release sulfur dioxide into the atmosphere . Encyclopædia Britannica, Inc. Sulfuric acid is a very strong acid; in aqueous solutions it ionizes completely to form hydronium ions ...
What is sulfuric acid used for?
In various concentrations the acid is used in the manufacture of fertilizers, pigments, dyes, drugs, explosives, detergents, and inorganic salts and acids, as well as in petroleum refining and metallurgical processes. In one of its most familiar applications, sulfuric acid serves as the electrolyte in lead –acid storage batteries.
What is fuming sulfuric acid?
The term fuming sulfuric acid, or oleum, is applied to solutions of sulfur trioxide in 100 percent sulfuric acid; these solutions, commonly containing 20, 40, or 65 percent sulfur trioxide, are used for the preparation of organic chemicals.
What is hydrogen sulfate?
See Article History. Alternative Titles: hydrogen sulfate, oil of vitriol, sulphuric acid. Sulfuric acid, sulfuric also spelled sulphuric (H2SO4), also called oil of vitriol, or hydrogen sulfate, dense, colourless, oily, corrosive liquid; one of the most commercially important of all chemicals.
What temperature does sulfuric acid boil?
This mixture of sulfuric acid and water boils at a constant temperature of 338 °C (640 °F) at one atmosphere pressure.
How is sulfuric acid produced?
There are four main process steps in the production of sulfuric acid from sulfur dioxide -containing gases by the contact process: 1) Gas drying 2) Catalytic conversion of sulfur dioxide to sulfur trioxide, 3) Absorption of sulfur trioxide and 4) Acid cooling.
What bacteria use sulfur to make sulfuric acid?
Sulfur that is in water may be oxidized to sulfuric acid by sulfur bacteria (Thiobacilli) that use sulfur to obtain energy for growth (1). (1) ATSDR; Toxicological Profile for Sulfur Trioxide and Sulfuric Acid. Atlanta, GA: Agency for Toxic Substances and Disease Registry, US Public Health Service (1998).
What is sulfuric acid mist?
In particular, sulfuric acid mists may be produced during the manufacture or use of sulfuric acid, sulfur trioxide, or oleum.
How does sulfuric acid release to the environment?
Sulfuric acid's production and use in the manufacture of fertilizers, explosives, dyestuffs and other chemicals (1) and in petroleum refining, ore processing, paper manufacture and other uses (1,2) may result in its release to the environment through various waste streams (SRC). Sulfuric acid droplets can form in the air when sulfur dioxide is released from the burning of coal, oil, and gas; the released sulfur dioxide slowly forms sulfur trioxide and then reacts with water in the air to form sulfuric acid (2). The occurrence of sulfuric acid in the environment comes mainly from the hydrolysis of sulfur oxides produced by combustion processes (natural and anthropogenic), wet deposition, generally as a mixture with nitrogen oxides and nitric acid and not from the manufacturing and use of the acid (3). In some countries, sulfuric acid is approved for agricultural use (3) which will release the compound directly to the environment (SRC).
What is the USEPA code for sulfuric acid?
For sulfuric acid (USEPA/OPP Pesticide Code: 078001) ACTIVE products with label matches. /SRP: Registered for use in the U.S. but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved uses./
What is the rate constant of sulfuric acid in the atmosphere?
A rate constant of 1.5X10-14 cu cm/molecule-sec at 25 °C was approximated for the gas-phase reaction of hydroxyl radicals with a single hydrogen atom of sulfuric acid from the HSO4 radical; it was suggested that this reaction could be a sink for gaseous sulfuric acid in the atmosphere (1). Sulfuric acid in the atmosphere can react with other compounds to form sulfate aerosol particulates; for example, if ammonia is present, sulfuric acid will react to form ammonium sulfates (2).
What are the effects of sulfuric acid aerosols?
People at particular risk were those with pre-existing cardiovascular and pulmonary disease. Similar gas-aerosol complexes have been responsible for acute and chronic lung disease, including potentiation of respiratory tract infections and chronic bronchitis in geographical areas where there is significant air pollution from stationary sources of fossil fuel combustion. Although sulfuric acid is only one component of these complexes, it has been suggested that hydrogen ion concn, presumably primarily reflecting sulfuric and nitric acids, is correlated with bronchitic symptoms in children. Levels of sulfate and fine particles may also be better predictors of mortality than are concentrations of total suspended particles or inhalable particles.
What are some products made with sulfuric acid?
Hydrochloric acid, nitric acid, detergents, pigments and sulfate salts are all made using sulfuric acid, and rayon and over-the-counter medications can also contain sulfuric acid. Advertisement. Household Uses for Sulphuric Acid. Image Credit: David Kashakhi/iStock/GettyImages.
What household products contain sulfuric acid?
Household Products Containing Sulfuric Acid. Sulfuric acid is commonly found in household cleaning products, though it is not limited to that use. The reason that sulfuric acid household products are so common has to do with its corrosive properties. This makes it an excellent choice for products such as toilet bowl cleaners ...
Why do drain cleaners contain sulfuric acid?
In addition, most drain cleaners and toilet bowl cleaners contain sulfuric acid because of its ability to break down organic components such as hair.
What happens when sulfuric acid batteries are used?
Because of this, all sulfuric acid batteries have a lifespan of use, and you will get diminishing returns with the battery until it is spent.
What is sulfuric acid used for?
When used in the production of fertilizers, the superphosphate of lime is mixed with ammonium sulfate. Hydrochloric acid, nitric acid, detergents, pigments and sulfate salts are all made using sulfuric acid, ...
Why is sulfuric acid used in batteries?
Sulfuric acid has countless potential uses. Because it is easily diluted, it can be used for anything from skin care to construction jobs. In addition, you can use sulfuric acid when you need to cause a specific chemical reaction. For this reason, sulfuric acid is a major component in various sorts of batteries.
What to do if you eat sulfuric acid?
If sulfuric acid is ingested, do not attempt to throw up. Contact emergency services as soon as possible. To make certain that you will get the correct treatment, take a picture of the container or bring it with you to the emergency department if at all possible. You can also contact poison control at 800-222-1222 for other instructions.
What is Sulfuric Acid?
Sulfuric acid is a mineral liquid that is used to make hundreds of compounds in the chemical industry.
Sulfuric Acid Chemical Properties: Solubility, Molar Mass and Molecular Density
Sulfuric acid (H₂SO₄) is a colorless, mineral liquid that contains hydrogen, sulfur, and oxygen.
What is Sulfuric Acid Used For?
Sulfuric acid has many uses among a broad expanse of industries and is one of the most important industrial chemicals. In almost any manufactured good, the use of sulfuric acid could be traced back to some step of the manufacturing process.
What are the sources of sulfur?
Allium Vegetables. Other than proteins, allium vegetables are one of the main sources of dietary sulfur. This group of vegetables is rich in various forms of sulfur, including sulfides, thiosulfates, sulfoxides, vinyldthiins, and ajoenes.
What foods contain sulfur?
However, some consumers have reported experiencing gastrointestinal discomfort, with cases resulting in ulcerative colitis from sulfates found in drinking water and some allium and cruciferous vegetables. Turkey, beef, eggs, fish, and chicken.
What are the two amino acids that make proteins?
In this Article. Sulfur is the third most abundant element in your body. It is present in methionine and cysteine , which are two of the amino acids you use to make proteins. Both of these amino acids are present in your skin, hair, and nails where they help to make these tissues strong and flexible.
What are the nutrients in cruciferous vegetables?
The cruciferous group of vegetables includes broccoli, cauliflower, cabbage, arugula, kale, and radishes. Whole grains are a good source of sulfur in the form of thiamin (vitamin B-1). Like the essential amino acid methionine, thiamine cannot be produced by your body and must be obtained from your diet.
Why do we need sulfur?
Why You Need Sulfur. Your body needs sulfur to build and fix your DNA and protect your cells from damage that can lead to serious diseases such as cancers . Sulfur also assists your body to metabolize food and contributes to the health of your skin, tendons, and ligaments. The two amino acids that include sulfur are methionine and cysteine.
What are some good sources of cysteine?
Nuts, seeds, grains and legumes are great plant-based sources of this amino acid. Chickpeas, couscous, eggs, lentils, oats, turkey and walnuts are good sources of getting cysteine through your diet. Other than proteins, allium vegetables are one of the main sources of dietary sulfur.
What foods contain methionine?
Turkey, beef, eggs, fish, and chicken. Turkey, beef, eggs, fish, and chicken are animal-based sources of methionine, the essential amino acid that must be consumed through your diet since it cannot be synthesized by your body. Nuts, seeds, grains, and legumes. It is also possible to obtain methionine from a vegetarian diet.
Overview
- Sulfuric acid is in numerous household cleaners, particularly toilet bowl cleaners and drain de-cloggers. It is also in some powdered laundry detergents, pet-care products, rust-dissolving solutions, metal cleaners, hand soaps, dish-washing liquids and rain repellents used to prevent streaking on automobiles as well as some products associated with...
Occurrence
Physical properties
Chemical properties
Manufacture
Pure sulfuric acid is not encountered naturally on Earth in anhydrous form, due to its great affinity for water. Dilute sulfuric acid is a constituent of acid rain, which is formed by atmospheric oxidation of sulfur dioxide in the presence of water – i.e. oxidation of sulfurous acid. When sulfur-containing fuels such as coal or oil are burned, sulfur dioxide is the main byproduct (besides the chief p…
Uses
Although nearly 100% sulfuric acid solutions can be made, the subsequent loss of SO3 at the boiling point brings the concentration to 98.3% acid. The 98.3% grade is more stable in storage, and is the usual form of what is described as "concentrated sulfuric acid". Other concentrations are used for different purposes. Some common concentrations are:
"Chamber acid" and "tower acid" were the two concentrations of sulfuric acid produced by the lea…
History
Because the hydration reaction of sulfuric acid is highly exothermic, dilution should always be performed by adding the acid to the water rather than the water to the acid. Because the reaction is in an equilibrium that favors the rapid protonation of water, addition of acid to the water ensures that the acid is the limiting reagent. This reaction is best thought of as the formation of hydronium
Safety
Sulfuric acid is produced from sulfur, oxygen and water via the conventional contact process (DCDA) or the wet sulfuric acid process (WSA).
In the first step, sulfur is burned to produce sulfur dioxide.
S(s) + O2 → SO2
The sulfur dioxide is oxidized to sulfur trioxide by oxygen in the presence of a vanadium(V) oxide