In the test measures:
- pH: The pH tells you if your patient is acidotic or alkalotic. It is a measurement of the acid content or hydrogen ions [H+] in the blood. ...
- PCO2: The PaCO2 level is the respiratory component of the ABG. ...
- Bicarbonate (HCO3): level is the metabolic component of the ABG. ...
- Total CO2 Contents (TCO2): ...
- Standard Bicarbonate (SBC): ...
- Base Excess: ...
- PO2: ...
- Oxygen saturation capacity: ...
See more

What do you test for hydrogen?
A characteristic test for hydrogen (H2) gas can be performed by bringing a burning candle near the source of hydrogen. On doing so, hydrogen gas burns with a squeaky pop sound. Hydrogen gas is recognized by the 'pop' when it burns. The 'pop' is the sound of a small explosion.
What are the properties of hydrogen?
Physical properties of Hydrogen: The hydrogen gas is colourless, odourless and tasteless in nature. It is a combustible gas but not a supporter of combustion. It is lighter than air and insoluble in water. It has an atomic mass of 1.008 amu and an ionization enthalpy of 1312 kJ mol-1.
What test is used to identify the presence of hydrogen gas?
splint testBurning (or lighted) splint test If the gas is flammable, the mixture ignites. This test is most commonly used to identify hydrogen, which extinguishes with a distinctive 'squeaky pop' sound. Hydrogen is easily ignited and used to definitively conclude what the gas actually is.
What are the tests for hydrogen and oxygen?
Hydrogen (H2) When a burning splint is introduced to a sample of pure hydrogen gas, it will burn with a popping sound. Oxygen (O2) When a smoldering splint is introduced to a sample of pure oxygen gas, the splint will reignite.
What are the four properties of hydrogen gas?
Hydrogen is a colorless, odorless, tasteless, and nonpoisonous gas under normal conditions on Earth.
What are 5 facts about hydrogen?
Hydrogen FactsHydrogen is the most abundant element. ... There are three natural isotopes of hydrogen: protium, deuterium, and tritium. ... Hydrogen gas is extremely flammable. ... Hydrogen compounds commonly are called hydrides.Hydrogen may be produced by reacting metals with acids (e.g., zinc with hydrochloric acid).More items...•
What are the two tests of hydrogen?
Characteristic test for hydrogen (H2) gas can be performed by bringing a burning candle near the source of hydrogen. On doing so, hydrogen gas burns with a squeaky pop sound. Hydrogen gas is recognised by the 'pop' when it burns. The 'pop' is the sound of a small explosion.
What are the 3 gas tests?
SummaryTestObservationInferenceLighted splint held in a test tubePop sound heardHydrogen is presentGas bubbled through limewaterLimewater turns milky or cloudy whiteCarbon dioxide is presentDamp litmus paper held in a test tubePaper turns whiteChlorine is present1 more row
How do you test for hydrogen water?
A simple titration (oxidimetry) method using a methylene blue-platinum colloid reagent is effective in determining the concentration of hydrogen gas in an aqueous solution. The method performs as effectively as the more complex and expensive electrochemical method.
What are the 10 chemical properties of hydrogen?
Let us look at some of the important physical and chemical properties of hydrogen.Hydrogen has an atomic number of 1.It has an atomic weight of 1.0080.The ionization potential of hydrogen is given as 13.595 electron volts.Its electron affinity is 0.7542 electron volts.Its nuclear spin is ½More items...
What are the properties of hydrogen for kids?
Hydrogen is the lightest of the elements and is the most abundant element in the universe. At standard temperature and pressure hydrogen is a colorless, odorless, and tasteless gas. Hydrogen is very flammable and burns with an invisible flame. It burns when it comes into contact with oxygen.
What are the three properties of hydrogen bonds?
The conditions for hydrogen bonding are:The molecule must contain a highly electronegative atom linked to the hydrogen atom. The higher the electronegativity more is the polarization of the molecule.The size of the electronegative atom should be small. The smaller the size, the greater is the electrostatic attraction.
What are 5 common uses of hydrogen?
The Many Uses of HydrogenRenewable Energy Storage. Solar and wind power. ... Power Generation. ... Stationary Fuel Cells. ... Power to Gas. ... Methanation.
What are the properties of hydrogen sulfide?
Hydrogen sulfide is colourless, poisonous gas having the odour of a rotten egg. It is produced by the decomposition of organic matter by microorgan...
What are the properties of hydrogen chloride?
Hydrogen chloride is a gas with a pungent odour and has a pale yellow colour. It is diatomic and covalent.
What are the five physical properties of hydrogen?
Hydrogen is colourless, odourless, tasteless, non-metallic diatomic gas. It is a highly combustible gas.
What are the properties and uses of hydrogen?
Hydrogen is colourless, odourless, tasteless, non-metallic diatomic gas. It shows both electropositive and electronegative properties. It has high...
What are the two properties of pure hydrogen?
Pure hydrogen exists as a dihydrogen molecule. It is a highly combustible gas.
What are the physical and chemical properties of hydrogen?
The physical properties of hydrogen: It is a colourless, odourless, tasteless, non-metallic diatomic gas. The chemical properties of hydrogen: It s...
How many protons and electrons are in hydrogen?
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for Hydrogen is H.
How many protons does helium have?
Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure. The chemical symbol for Helium is He.
What is the charge of an atom?
Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. In a neutral atom there are as many electrons as protons moving about nucleus. It is the electrons that are responsible for the chemical bavavior of atoms, and which identify the various chemical elements.
How is atomic weight determined?
Therefore it is determined by the mass number (number of protons and neutrons).
What is the Pauli exclusion principle?
It is the Pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. The ordering of the electrons in the ground state of multielectron atoms, starts with the lowest energy state (ground state) and moves progressively from there up the energy scale until each of the atom’s electrons has been assigned a unique set of quantum numbers. This fact has key implications for the building up of the periodic table of elements.
How are the chemical properties of a solid, liquid, gas, and plasma determined?
The chemical properties of the atom are determined by the number of protons, in fact, by number and arrangement of electrons. The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z.
What is the density of a substance?
Since the density (ρ) of a substance is the total mass (m) of that substance divided by the total volume (V) occupied by that substance, it is obvious, the density of a substance strongly depends on its atomic mass and also on the atomic number density (N; atoms/cm 3 ),
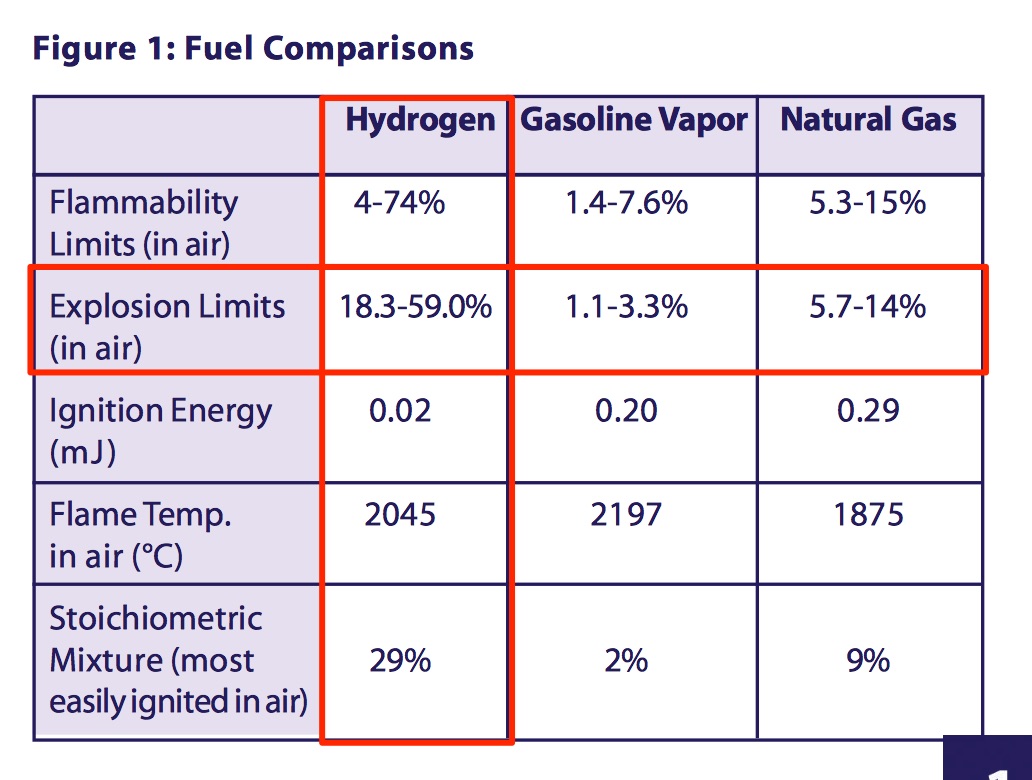
Hydrogen is Flammable and Ignites easily
Designers and operators of hydrogen systems should be aware that hydrogen's flammability range is very wide compared to other fuels.
Leaks are Hard to Detect
Hydrogen is colorless and odorless, so human senses can't detect it. Personnel should use caution when approaching an area where there is the potential for a hydrogen leak. Best practices include the following:
Flames are Hard to See
Hydrogen burns with a pale blue flame that is nearly invisible in daylight. The flame may appear yellow if there are impurities in the air (e.g., dust or sodium from ocean spray). A pure hydrogen flame will not produce any smoke. Hydrogen flames also emit low radiant heat, so a person may not feel heat until they are very close to the flame.
Hydrogen is Buoyant
Hydrogen gas is much more buoyant that other gases, so when it leaks into air, it will rise rapidly. This is an advantage for hydrogen systems installed outdoors because the leaking hydrogen is rapidly dispersed. In enclosed spaces, leaking hydrogen will accumulate in the ceiling pockets, potentially creating an explosive mixture.
Summary
This project develops measurements, modeling, and science relevant to the effect of hydrogen on materials. Mechanical measurements focus on the fatigue and fracture properties of pipeline and pressure vessel steels.
Description
Hydrogen is known to have a deleterious effect on steels and other metals, but steels are the most cost-effective and commonly used materials for pipelines and pressure vessels.
Major Accomplishments
Our group has developed a test apparatus (for which we received a patent in 2015) and method that simultaneously measures 10 specimens at once, and maintains specific environmental and loading conditions until all 10 specimens have completed testing.
Recent Publications
Amaro RL, Rustagi, N., Findley, KO, Drexler, ES, Slifka, AJ (2014) Modeling the fatigue crack growth of X100 Pipeline Steels in Gaseous Hydrogen. International Journal of Fatigue, 59, 262-271.
What is the SAE J2579?
Additional standards are being developed and validated (SAE J2579, ISO 15869) to further improve and validate safety standards for high-pressure hydrogen tanks. Vehicle manufacturers (domestic and foreign) are working closely together with tank manufactures and others to develop robust test procedures to ensure the safety of the tanks and the entire vehicle.
What is a high pressure hydrogen tank?
Improved versions of these tanks made of high-strength composite materials are now used to store hydrogen at higher pressures (5,000 and 10,000 psi) to achieve greater driving range in hydrogen-fueled vehicles. High-pressure hydrogen tanks are designed not to rupture and are held to rigorous performance requirements. Furthermore, these tanks undergo extensive testing to make sure that they meet these performance requirements. A table of standards enacted or under development and various required tests are shown in Table 1.
How many high pressure composite tanks are there?
Worldwide, it has been estimated that millions of high-pressure composite tanks are in use in various commercial and industrial applications, and the overall safety record of these tanks has been excellent.
Why are tanks exposed to pressures above normal?
Tanks are exposed to pressures above normal to simulate fault management. The tanks are also dropped 6 feet when empty, shot with a rifle, burned, and exposed to acids, salts, and other road hazards to validate that they are safe even under severe or unusual conditions.
Is a hydrogen tank certified?
Many types of compressed hydrogen tanks have been certified worldwide and demonstrated in several prototype fuel cell vehicles. The following information discusses high-pressure hydrogen tank testing, codes and standards, and certifications.
Can composite tanks be removed from service?
Safety. In the unlikely case that an advanced composite tank le aks, it can be removed from service without incident. It is highly unlikely that these tanks will fail in a way that will directly endanger the occupants of a hydrogen-fueled vehicle.
Do hydrogen filling stations have overpressure protection?
At the end of life they are tested to approximately twice their working pressure, according to the Society of Automotive Engineers (SAE), Journal 2579. In addition, hydrogen filling stations have numerous redundant overpressure protection systems so that it is not possible to over-pressurize a vehicle fuel system.
Why is hydrogen in gas so difficult to extract?
And because hydrogen in gas form is highly flammable, the issue of consumer safety is also a factor.
Is hydrogen a renewable fuel?
Today, there's more and more emphasis on using fuels that are renewable and that don't produce harmful emissions. Hydrogen is one such fuel, but the technology to use it still needs work. Learn more in this lesson.
What do my results mean?
If you test negative, it’s possible that you have dysbiosis lower down in your large intestine (also known as your colon), or a fungal overgrowth, which doesn’t show up on SIBO breath tests.
Why is it important to do a SIBO test?
It’s very important that your SIBO test analyses both hydrogen and methane levels, because both gases are involved in SIBO. If your test only looks for hydrogen, you could easily get a false negative.
How long does it take to get a SIBO test?
At Healthpath, we have a simple and fast SIBO home test. It also takes three hours, but you don’t have to travel anywhere to do it. It measures both hydrogen and methane levels: two gases known to cause SIBO symptoms.
What is SIBO in IBS?
If you’ve got IBS symptoms, and you’ve been trying to find out why, the chances are you’ve come across SIBO (small intestinal bacterial overgrowth) as a possible root cause.
Why do we use hydrogen breath test for SIBO?
Leading practitioners use the hydrogen/methane breath test for SIBO because overall, it gives the most accurate and reliable results.
How much does a SIBO test cost?
At-home tests are usually cheaper. Our at-home SIBO test at Healthpath is £165, which includes bespoke advice from our trained and experienced health professionals, and a protocol designed to get to the root cause of your symptoms.
How to take a hydrogen breath test?
To take a hydrogen breath test, you’ll need to drink a sugary solution called lactulose, after eating a special diet then fasting the day before. You’ll then breathe into bags supplied with the test kit every 20 minutes for three hours afterwards.
