
What is the equation for alcohol dehydration?
A basic equation for alcohol dehydration is C 2 H 5 OH C 2 H 4 + H 2 O Alcohol dehydration is an example of an elimination reaction. An elimination reaction is the type of reaction in which two atoms adjacent to carbon atoms are eliminated from a molecule leaving multiple bonds between the carbon atoms.
Is alcohol dehydration an example of an elimination reaction?
Alcohol dehydration is an example of an elimination reaction. An elimination reaction is the type of reaction in which two atoms adjacent to carbon atoms are eliminated from a molecule leaving multiple bonds between the carbon atoms.
What type of alcohols are easy to dehydrate?
Tertiary alcohols are easy to dehydrate but on the other hand, primary alcohol dehydration is very tough.
What is the rate-determining step in the dehydration of alcohols?
Hence, the formation of the carbocation is considered to be the rate-determining step. This is the ultimate step in the dehydration of alcohols. Here, in this step, the generated proton is eliminated with the help of a base.
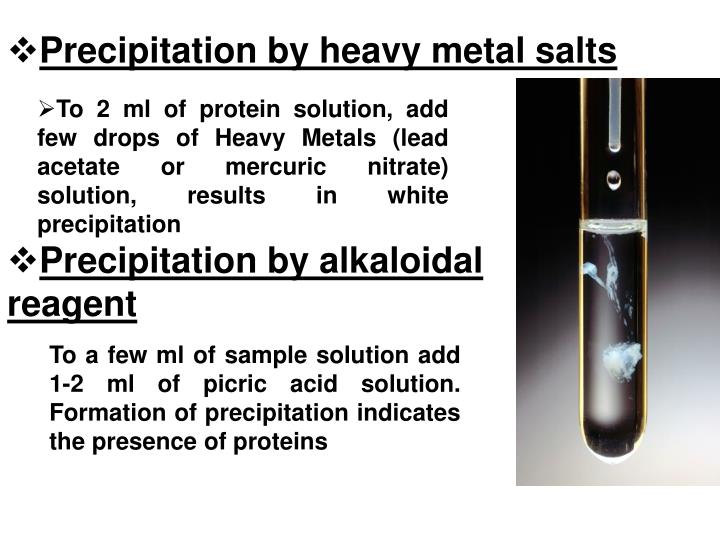
What reagent is used for alcohol?
If the alcohol is primary or secondary, the reagent of choice is phosphorous tribromide (PBr3). If the alcohol is tertiary, we use hydrogen bromide (HBr) to ake the alkyl halide.
Which reagent is used for dehydration?
The Burgess reagent is used to convert secondary and tertiary alcohols with an adjacent proton into alkenes. Dehydration of primary alcohols does not work well. The reagent is soluble in common organic solvents and alcohol dehydration takes place with syn elimination through an intramolecular elimination reaction.
How is alcohol prepared by dehydration method?
The dehydration of ethanol to give ethene Ethanol is heated with an excess of concentrated sulphuric acid at a temperature of 170°C. The gases produced are passed through sodium hydroxide solution to remove the carbon dioxide and sulphur dioxide produced from side reactions.
What is dehydration product of alcohol?
Dehydration of alcohol is defined as a reaction in which alcohol reacts with protic acid to lose water molecules in the presence of heat and form alkenes.The general reaction of dehydration of alcohol can be represented as: Acid catalyzed dehydration of alcohol occurs via an E1 mechanism.
Why is sulfuric acid used in dehydration of alcohols?
Concentrated sulphuric acid is used for the dehydration of alcohol because sulphuric acid is a strong oxidizing agent. It also has a strong affinity toward water thus absorbing water. When concentrated sulphuric acid reacts with alcohol, it oxides some alcohol to carbon dioxide and reduces itself to sulphur dioxide.
What is used for dehydration?
The treatment for dehydration is to replace the fluids and electrolytes that you have lost. For mild cases, you may just need to drink lots of water. If you lost electrolytes, sports drinks may help. There are also oral rehydration solutions for children.
Which is used commonly in the dehydration of water?
In chemical reactions where dehydration occurs, the reacting molecule loses a molecule of water. Sulfuric acid, concentrated phosphoric acid, hot aluminum oxide, and hot ceramic are common dehydrating agents in these types of chemical reactions.
Which technique is used for dehydration of ethanol?
Ethyl alcohol is concentrated and dehydrated by a distillation process.
Is hydrochloric acid a dehydrating agent?
At 98% concentration, nitric and perchloric acids are likely to be very good dehydrating agents, but these are also very strong oxidizers, less stable, etc. Likewise, commercial hydrochloric acid is sold as a 38% solution in water, so it already has had its dehydrating ability consumed.
Why is acid required for dehydration of alcohols?
Acid-catalyzed dehydration of alcohols occurs when an acid protonates the alcohol, creating water as a good leaving group. When water leaves a molecule, this is called a dehydration reaction. Dehydration of alcohols results in the formation of an alkene (double bond).
What is alcohol dehydration organic chemistry?
The elimination of a hydroxyl group in an alcohol along with the hydrogen from an adjacent carbon can yield an alkene. As a molecule of water is lost, this is a dehydration reaction. Alcohols are commonly dehydrated by heating in the presence of an acid catalyst.
Which alcohol is most easily dehydrated?
The alcohol that is dehydrated most easily with conc. H2SO4 is p−CH3OC6H4CH(OH)CH3.
Which reagent is used for dehydration of carboxylic acids?
NaBH4 or Ni/H2.
Would you give d5ns for dehydration?
Note #3: The dehydration component of fluid replacement MUST be provided as 0.9% saline. NEVER use a hypotonic saline, such as D5 0.18% (fifth-normal saline), D5 0.3% (third-normal saline) or even D5 0.45% (half-normal saline) to correct dehydration.
What shows dehydration in urine test?
High specific gravity suggests that the concentration of urine is too high. This can be a sign of dehydration, and the doctor may recommend drinking more clear fluids. Conditions that cause high specific gravity include: dehydration.
How do you give fluid for dehydration?
Give oral rehydration solution (ORS) immediately to dehydrated patients who can sit up and drink. If ORS is not available, you should provide water, broth, and/or other fluids. You should not provide drinks with a high sugar content, such as juice, soft drinks, or sports drinks, because they could worsen diarrhea.
What happens when an aldehyde is introduced to the Tollens reagent?from byjus.com
When an aldehyde is introduced to the Tollens reagent, two things occur: The aldehyde is oxidized by the Tollens reagent and forms a carboxylic acid. This reaction can be written as follows: The silver ions present in the Tollens reagent are reduced into metallic silver.
What is Tollens’ Reagent?from vedantu.com
Tollens’ reagent which was initially discovered by a German chemist Bernhard Tollens is the chemical reagent that is used in the Tollen’s test to determine the presence of aldehyde functional groups along with some alpha-hydroxy ketones in an unknown solution. It is a colourless, aqueous solution consisting of silver ammonia complex in ammonia solution. Sodium hydroxide present in the solution maintains a basic pH i.e. (pH>7) of the solution.
What is the purpose of the tollen test?from vedantu.com
A tollen test is performed to determine the presence of an aldehyde or a ketone in a given unknown solution. This test is named after the German chemist Bernhard Tollens and is also known as Silver-Mirror Test. Carbonyl compounds are the compounds which contain >C=O as their functional group. These can be either:
What gives a grey black precipitate?from vedantu.com
Aldehyde gives a grey black precipitate or a silver mirror when freshly prepared Tollens’ reagent is added to the solution.
Why is the Tollens test positive?from vedantu.com
Some reducing sugars or carbohydrates do not have an aldehydic group but they also give Tollens’ test as positive because of isomerization in the alkaline medium and such sugars are also categorized as reducing sugars. Therefore tollens’ reagent is used in the identification and differentiation of carbohydrates/sugars on the basis ...
What is reducing sugar?from vedantu.com
Carbohydrates are basically Polyhydroxy Aldoses or Ketoses. There are several carbohydrates which have a free aldehyde group and such sugars easily reduce Tollens’ reagent, Fehling’s reagent or Benedict’s solution and are therefore called reducing sugars.
Why does Tollens have a mild pH?from vedantu.com
Tollens’ reagent has a mild basic pH because of the presence of NaOH however it isn’t corrosive in nature . So, basic laboratory safety measures are enough while handling those chemicals.
What is reagent grade alcohol?
What is reagent grade alcohol? Reagent grade alcohol is a strong, laboratory grade alcohol used for a variety of scientific purposes. If you need more help to better understand reagent grade alcohol, and if it is right for you, contact Ecolink here!
What is Ecolink's expert advice?
Expert Advice – Ecolink and their knowledgeable team of experts are happy to work closely with their clients, to ensure they are purchasing the best chemicals for their particular needs, in addition to fully understanding the chemical products they purchase.
