Stabilizing bonds in protein structure
- 1. The covalent bonds These strong bonds result from the sharing of electrons between two atoms. There are four different types. Polypeptide Peptide linkage The peptide bond -CO-NH-bond is responsible for forming the carbon skeleton of the primary structure of proteins. ...
- 2. The Non-Covalent Bonds
What are the interactions that stabilize each level of protein structure?
What are the main interactions that stabilize each level of protein structure? The primary structure is held together by covalent peptide bonds. They are formed during the process of protein biosynthesis, where the amino acids lose one water molecule per reaction to attach to another amino acid.
What is the primary and secondary structure of a protein?
The primary structure is held together by covalent peptide bonds. They are formed during the process of protein biosynthesis, where the amino acids lose one water molecule per reaction to attach to another amino acid. The secondary structure is determined by hydrogen bonds between the main-chain peptide groups.
How do you enhance the structure of proteins?
In the interior of the protein there is very little space, which leads to structural enhancement from London dispersion forces, which result from the tight packing (7)*. Protein architecture can also be enhanced by disulfide bonds between two amino acids that have sulfur components.
How do hydrophobic and hydrogen bonds affect protein stability?
Hydrogen and hydrophobic interactions play very active roles in maintaining protein stability (4, 5). In hydrogen interactions, the nitrogen and oxygen atoms in the peptides form hydrogen bonds that contribute to the configuration of a protein’s three-dimensional structure (6).
What stabilizes the primary structure?
The primary structure is held together by covalent peptide bonds. They are formed during the process of protein biosynthesis, where the amino acids lose one water molecule per reaction to attach to another amino acid.
Why are primary protein structures stable?
The side-chain substituents of the amino acid groups in an α-helix extend to the outside. Hydrogen bonds form between the oxygen of each C=O. bond in the strand and the hydrogen of each N-H group four amino acids below it in the helix. The hydrogen bonds make this structure especially stable.
What stabilizes secondary protein structure?
Secondary structure elements that are formed early in protein folding (15,16) are stabilized by both sequence-dependent side-chain interactions and sequence-independent backbone interactions (particularly hydrogen bonding).
Do van der Waals forces stabilize proteins?
This suggests that van der Waals interactions make an important contribution to the stability gained by proteins when nonpolar groups are buried. Several other lines of reasoning have led to the same conclusion [30–32].
How are protein structures stabilized?
Folded proteins are stabilized by thousands of noncovalent bonds between amino acids. In addition, chemical forces between a protein and its immediate environment contribute to protein shape and stability.
What stabilizes the tertiary structure of proteins?
The tertiary structure of a protein refers to the overall three-dimensional arrangement of its polypeptide chain in space. It is generally stabilized by outside polar hydrophilic hydrogen and ionic bond interactions, and internal hydrophobic interactions between nonpolar amino acid side chains (Fig. 4-7).
What bonds stabilize the secondary structure?
Secondary structure refers to regular, recurring arrangements in space of adjacent amino acid residues in a polypeptide chain. It is maintained by hydrogen bonds between amide hydrogens and carbonyl oxygens of the peptide backbone.
How do disulfide bonds stabilize proteins?
Classical theory suggests that disulfide bonds stabilize proteins by reducing the entropy of the denatured state. More recent theories have attempted to expand this idea, suggesting that in addition to configurational entropic effects, enthalpic and native-state effects occur and cannot be neglected.
What describes the primary structure of a protein?
To reiterate, the primary structure of a protein is defined as the sequence of amino acids linked together to form a polypeptide chain. Each amino acid is linked to the next amino acid through peptide bonds created during the protein biosynthesis process.
How does the primary structure of protein differ from secondary?
While primary structure describes the sequence of amino acids forming a peptide chain, secondary structure refers to the local arrangement of the chain in space. Several common secondary structures have been identified in proteins.
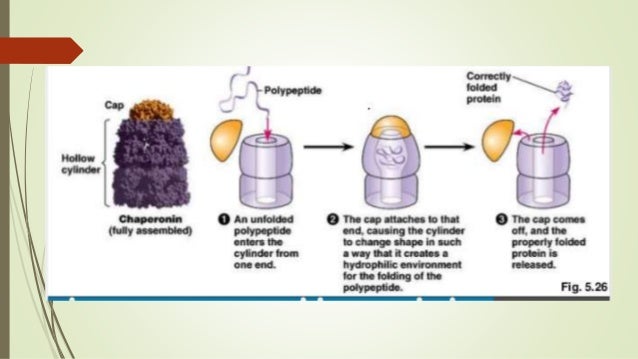
How do proteins adopt and maintain a stable folded structure?
Hydrogen bonds in a protein molecule. Large numbers of hydrogen bonds form between adjacent regions of the folded polypeptide chain and help stabilize its three-dimensional shape.
How does the primary structure of a protein affect the other structural levels?
Explanation: The primary structure of a protein is defined by the sequence of amino acid residues. It is this sequence that lays the foundation for all other higher levels of structures in a protein. Secondary structure is defined by the hydrogen bonding between the carboxyl and amino backbone of the amino acids.
Which amino acid clusters on the inside of a protein?
Also important to tertiary structure are hydrophobic interactions, in which amino acids with nonpolar, hydrophobic R groups cluster together on the inside of the protein, leaving hydrophilic amino acids on the outside to interact with surrounding water molecules.
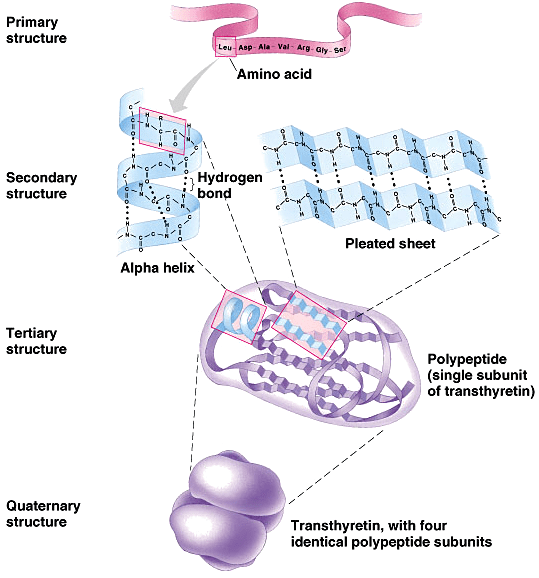
What are the four levels of protein structure?
To understand how a protein gets its final shape or conformation, we need to understand the four levels of protein structure: primary, secondary, tertiary, and quaternary.
How are the amino acids in insulin connected?
Image of insulin. Insulin consists of an A chain and a B chain. They are connected to one another by disulfide bonds (sulfur-sulfur bonds between cysteines). The A chain also contains an internal disulfide bond. The amino acids that make up each chain of insulin are represented as connected circles, each with the three-letter abbreviation of the amino acid's name.
How many polypeptide chains are there in insulin?
For example, the hormone insulin has two polypeptide chains, A and B, shown in diagram below. (The insulin molecule shown here is cow insulin, although its structure is similar to that of human insulin.) Each chain has its own set of amino acids, assembled in a particular order.
Where do the R groups of amino acids stick outward?
The R groups of the amino acids stick outward from the α helix, where they are free to interact. In a β pleated sheet, two or more segments of a polypeptide chain line up next to each other, forming a sheet-like structure held together by hydrogen bonds.
What happens when amino acids stick to one another?
The hydrophobic amino acids, trying to get away from the water surrounding them in the egg white, will stick to one another, forming a protein network that gives the egg white structure while turning it white and opaque. Ta-da! Thank you, protein denaturation, for another delicious breakfast.
What are the different order of proteins?
Orders of protein structure: primary, secondary, tertiary, and quaternary. Alpha helix and beta pleated sheet.
What are the stabilizing bonds in proteins?
Stabilizing bonds in protein structure. 1. The covalent bonds. These strong bonds result from the sharing of electrons between two atoms. There are four different types. Polypeptide Peptide linkage. The peptide bond -CO-NH-bond is responsible for forming the carbon skeleton of the primary structure of proteins.
Which region of a protein is buried within the molecule?
For example, in the soluble globular proteins, the hydrophobic region (the non-polar groups) is buried within the molecule, excluding water. This phenomenon stabilizes the tertiary structures.
What is the bridge between cysteines?
Characteristics of elastin are formed by lysyl oxidase. 2. The Non-Covalent Bonds. bonds in protein structure. a. The ionic bonds.
How do hydrogen bonds gain power?
Hydrogen bonds gain power when the three atoms are aligned on the same axis.
Do proteins act alone?
Proteins are vital macromolecules at both cellular and systemic levels, but they rarely act alone. Diverse essential molecular processes within a cell are carried out by molecular machines built from a large number of protein components organized by their Protein-Proteins interaction (PPI). Indeed, these interactions are at the core ...