What is the boiling point of water in the mountains?
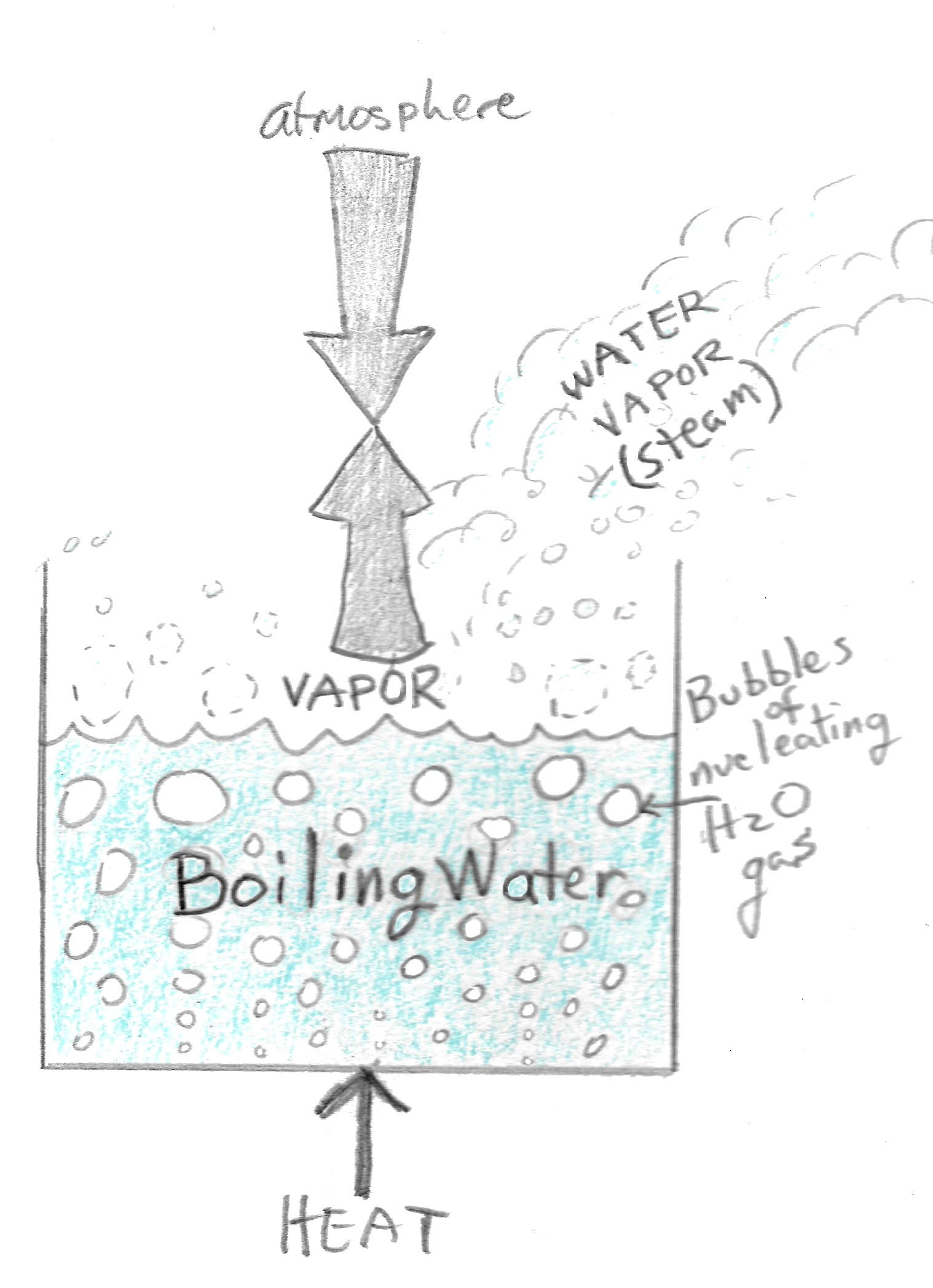
Click to see full answer. Also asked, what is the boiling point of water in the mountains? The boiling point of water varies with atmospheric pressure. At lower pressure or higher altitudes, the boiling point is lower. At sea level, pure water boils at 212 °F (100°C).
What temperature does water boil at high altitude?
At lower pressure or higher altitudes, the boiling point is lower. At sea level, pure water boils at 212 °F (100°C). At the lower atmospheric pressure on the top of Mount Everest, pure water boils at about 154 °F (68°C). Beside above, what temperature does water boil at high altitude? 212 °F
What temperature does water boil at the top of Mount Everest?
At the lower atmospheric pressure on the top of Mount Everest, pure water boils at about 154 °F (68°C). Also, what temperature does water boil at high altitude? 212 °F
What is the boiling point of water at sea level?
At sea level, atmospheric pressure is 14.7 psia and water boils at 212℉ (100℃). At an elevation of 8,000 feet (2,438 meters), water boils at 197℉ (91℃) and pressure is 10.9 psia. Click to see full answer.
Why does water boil at lower temperatures?
Why do you boil pasta longer?
Can you adjust bread machine recipes for high altitude?
Can you boil water at high altitude?
Can water boil at top of mountain?
At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F.
Why does water boil at 70 on Mount Everest?
When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude.
What temperature does water boil at 6000 feet elevation?
200.9FWater doesn't always boil at 100C/212F: For example, at 5,000 feet above sea-level water will boil at 94.9C (202.9F); at 6,000 feet water boils at 93.8C (200.9F); and at 7,000 feet water boils 92.7C (198.9F).
Can you hard boil an egg on Mt Everest?
This means at the top of Everest the boiling temperature of the water is 70°C. The reason you can not boil an egg is that an egg cooks at two different temperatures. The white cooks at 85°C and the yoke at 65°C. So on Everest, the whole of an egg will never cook.
What would happen if you boiled water on Mt Everest?
At lower pressure or higher altitudes, the boiling point is lower. At sea level, pure water boils at 212 °F (100°C). At the lower atmospheric pressure on the top of Mount Everest, pure water boils at about 154 °F (68°C).
What temperature does water boil in Texas?
212°FWhat is the Boiling Point of Water? Water boils at 212°F at sea level, but only at sea level.
What temperature does water boil at Lake Tahoe?
Boiling point. Water and other liquids evaporate faster and boil at lower temperatures. Hence, liquids boil at 212 degrees F at sea level and at Lake Tahoe at about 198 degrees F.
What temperature does water boil in Salt Lake City?
212 degrees(At sea level, water boils at 212 degrees.
Boiling Point at Altitude Calculator
The boiling point calculator tells you what temperature water will begin to boil at the given altitude. The altitude can be input in both metric and imperial units, and even nautical miles should that situation come up. Elevation matters because of some simple laws of basic physics we'll be explaining later on down.
Water Altitude Boiling Point Calculator - CSGNetwork
This calculator adjusts the boiling point based on Pressure or Altitude.
Why does water boil faster at the top of a mountain?
At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature.
Which can boil water faster at the mountain or in lowland Why?
At higher altitudes, air pressure is lower. … When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude.
Why is it difficult to boil water on a mountain?
The key factor is declining air pressure at higher altitudes. Falling air pressure lowers the boiling point of water by just under 1 degree Fahrenheit for each 500 feet of increased elevation. The lower boiling point means water will cook off more quickly, and at a lower temperature.
How long do you boil corn on the cob at high altitude?
How long to boil corn on the cob? The general rule is to boil corn for 10 minutes, however cooking time does vary based on altitude and also the size of your corn. You might need to boil a large ear it a little longer at 15 minutes or small ones shorter for 8 minutes.
Is it true that water boils at higher temperature at higher pressure?
When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. Therefore with higher atmospheric pressure, the boiling point would get higher.
Why water boils below 100 degree Celsius on the top of a mountain?
Water boils at a lower temperature on top of a mountain because there is less air pressure on the molecules. Higher the pressure, higher is the boiling point of water. At low pressure, the boiling point of water comes down. The boiling takes place at a lower temperature at the top of a mountain due to low pressure.
What temperature does water boil in Denver?
It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? Well, not always. It depends on where you’re doing the boiling. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations.
Why does water boil at lower temperatures?
As the altitude increases, the atmospheric pressure pushing down on water decreases, which allows the water to boil at lower temperatures. A lower boiling point means that food cooks at a lower temperature, despite the fact that the water is boiling. It is important to recognize just how much the temperature of boiling water is reduced as ...
Why do you boil pasta longer?
You would boil your food longer because it is boiling at a lower temperature. This means it takes pasta and rice longer to get done, and you may need to add more water to the pot as it boils off before they are the right consistency. But boiling isn't the only thing you need to pay attention to.
Can you adjust bread machine recipes for high altitude?
Each method of cooking, type of ingredients, and a combination of ingredients may require different tweaks to compensate for high altitude. As you can see in the chart, there is no one high altitude solution. For example, if you use a bread machine, you will need to adjust bread machine recipes for high altitude.
Can you boil water at high altitude?
High Altitude Cooking Tips. If you only had to worry about how boiling water is affected by altitude, cooking wouldn't be as much of a problem. Your pot of water will come to a boil sooner as it will boil at a lower temperature than at sea level.
