
Will regular salt melt ice?
You can use regular table salt to melt ice. It has properties which make it a great choice if you want to get rid of ice fast. Table salt has smaller granules so it is better able to dissolve into the ice. The faster a substance is able to break down, the quicker it succeeds in melting ice.
Why does rock salt make ice colder?
Why does rock salt make ice colder? On the surface of every ice cube, an exchange process goes on between water in the liquid and solid states. The presence of salt in the water lowers the equilibrium point of this exchange by lowering the freezing point of the liquid water. What kind of salt do you use to make ice cream?
Will Epsom salt melt ice?
Ice can be melted using Epsom salt, but it takes a long time. The chemical formula for Epsom salt is magnesium sulfate heptahydrate. Epsom salts are a more secure ice-melting agent than table salt. Plants and flora are not harmed by Epsom salts. They simply use the water available in snow or ice to remain hydrated.
Does salt melt when heated?
Does salt melt or burn? Some metals, like tin, do cause little explosions, but that's because their melting point is so low (231.9 degrees Celsius). But salt melts at around 800 degrees Celsius - almost 200 degrees higher than aluminium - which means that it should easily be hot enough to trigger the Leidenfrost effect.
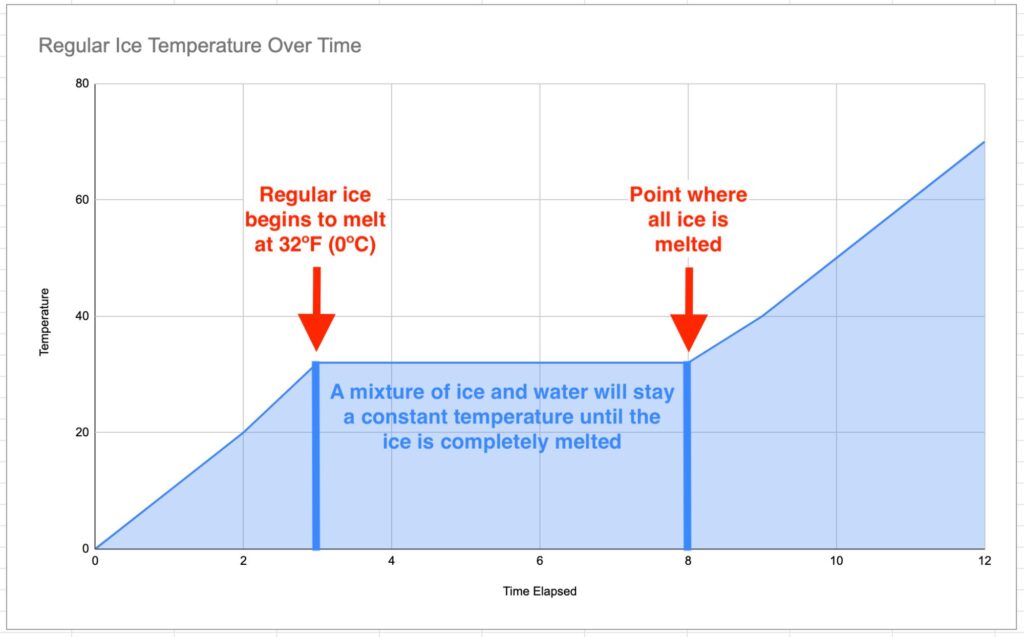
Adding Salt to Ice and Freezing Point Depression
Adding salt to ice doesn't just melt it. It also makes it colder because of freezing point depression. Dave King / Getty Images
Salt Lowers the Temperature of Ice Water
When you add salt to ice (which always has an outer film of water, so it's technically ice water), the temperature can drop from freezing or 0 °C to as low as -21 °C.
