What are the uses of threonine?
Aug 19, 2019 · The top threonine foods include: Organic meat (including chicken, lamb, beef and turkey) Wild-caught fish (including wild salmon) Dairy products. Cottage cheese. Eggs. Carrots. Bananas. Sesame seeds. Pumpkin seeds. Kidney beans.
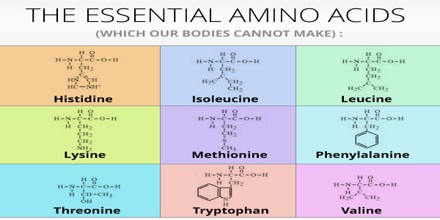
What are the 10 essential amino acids?
It is an enantiomer of a D-threonine. It is a tautomer of a L-threonine zwitterion. Threonine is an essential amino acid in humans (provided by food), Threonine is an important residue of many proteins, such as tooth enamel, collagen, and elastin. An important amino acid for the nervous system, threonine also plays an important role in porphyrin and fat metabolism and prevents …
What is threonine used for?
4 rows · Threonine, an essential amino acid, is a hydrophilic molecule. Threonine is an other ...
What does threonine do?
May 28, 2021 · Threonine is an amino acid that we use in the biosynthesis of proteins. It involves an α-amino group (a protonated − NH3+ structure under organic conditions), a carboxyl group (a deprotonated − COO− form under biological conditions), and a side chain containing a hydroxyl group (OH), making it a polar, uncharged amino acid.
Is threonine a polar amino acid?
Six amino acids have side chains that are polar but not charged. These are serine (Ser), threonine (Thr), cysteine (Cys), asparagine (Asn), glutamine (Gln), and tyrosine (Tyr). These amino acids are usually found at the surface of proteins, as discussed in the Proteins 2 module.
Is threonine an alpha amino acid?
It is an aspartate family amino acid, a proteinogenic amino acid, a threonine and a L-alpha-amino acid.
What functional group is threonine?
hydroxyl groupIt contains an α-amino group (which is in the protonated −NH+3 form under biological conditions), a carboxyl group (which is in the deprotonated −COO− form under biological conditions), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid.Jul 29, 2019
Is threonine semi essential amino acids?
The essential amino acids are isoleucine , leucine , lysine , methionine , phenylalanine , threonine, tryptophan, and valine . Another amino acid, histidine , is considered semi-essential because the body does not always require dietary sources of it.
Is threonine a protein?
Threonine is one of two proteinogenic amino acids with two stereogenic centers, the other being isoleucine.
What is the chemical structure of threonine?
D-threoninePubChem CID69435StructureFind Similar StructuresChemical SafetyLaboratory Chemical Safety Summary (LCSS) DatasheetMolecular FormulaC4H9NO3SynonymsD-threonine 632-20-2 (2R,3S)-2-amino-3-hydroxybutanoic acid H-D-Thr-OH Threonine, D- More...3 more rows
What is zwitterionic form of amino acid?
Under neutral conditions, the amino acid will exist in its zwitterion form. A zwitterion is a molecule that contains both a positive and a negative charge. For the zwitterion amino acid, the negative charge comes from the carboxylate ion while the positive charge comes from the ammonium ion.
What kind of isomers are produced by threonine?
Threonine has two chiral centers and therefore four possible stereoisomers. The isomers other than Thr (D-, L-allo-, and D-allo-threonine) are rare in nature and have no value, nutritional or otherwise. These days, most Thr is synthesized rather than being obtained from natural proteins.Jun 18, 2018
What is threonine found in?
2. ThreonineAnimal sources of threonine include lean beef, lamb, pork, collagen, gelatin, cheese. For every 100g of lean beef or lamb there's about 165% of your recommended dietary intake. ... Plant based sources include tofu, sunflower seeds, flaxseeds, wheat germ, cashews, almonds, lentils, and pistachios.Jun 8, 2020
What are 9 essential amino acids?
Essential amino acids cannot be made by the body. As a result, they must come from food. The 9 essential amino acids are: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.Nov 3, 2021
Which of the following amino acid is called as Semiessential amino acid?
The semi essential amino acids in humans are arginine and histidine.
Is threonine the same as theanine?
L-threonine vs L-theanine L-threonine is known to primarily affect the musculoskeletal system and the nervous system by improving cognitive functions like learning and memory. In contrast, L-theanine mainly works in the nervous system by promoting relaxation and providing calming effects.Aug 13, 2021
Q1: Explain the Two Best Threonine-Rich Foods.
Ans: the two threonine-rich foods are:TurkeyA chunk of protein-rich meat, for example, turkey has known to enough enhancement the body with threoni...
Q2: Explain the Role of Threonine on the Digestive System.
Ans: Threonine assumes a significant part in improving generally stomach related soundness of a person. It is related to delivering mucous gel laye...
Q3: List the Threonine Deficiency Disorders.
Ans: The deficiency of threonine must be treated with consuming a proper diet otherwise it may become very fatal and cause the following health iss...
What is L-threonine?
L-threonine is an optically active form of threonine having L-configuration. It has a role as a nutraceutical, a micronutrient, a Saccharomyces cerevisiae metabolite, a plant metabolite, an Escherichia coli metabolite, a human metabolite, an algal metabolite and a mouse metabolite. It is an aspartate family amino acid, a proteinogenic amino acid, ...
What is the role of threonine in the body?
An important amino acid for the nervous system, threonine also plays an important role in porphyrin and fat metabolism and prevents fat buildup in the liver.
How is nitrogen removed from amino acids?
Nitrogen is also removed from amino acids by deamination reactions, which result in the formation of ammonia. A number of amino acids can be deaminated, either directly ( histidine ), by dehydration ( serine, threonine), by way of the purine nucleotide cycle ( aspartate ), or by oxidative deamination ( glutamate ). ...
Is L-trinine a food additive?
L-Threonine is a food additive permitted for direct addition to food for human consumption, as long as 1) the quantity of the substance added to food does not exceed the amount reasonably required to accomplish its intended physical, nutritive, or other technical effect in food, and 2) any substance intended for use in or on food is of appropriate food grade and is prepared and handled as a food ingredient.
What are the amino acids in proteins?
The amino acids that are incorporated into mammalian protein are alpha-amino acids, with the exception of proline, which is an alpha-imino acid. This means that they have a carboxyl group, an amino nitrogen group, and a side chain attached to a central alpha- carbon. Functional differences among the amino acids lie in the structure of their side chains. In addition to differences in size, these side groups carry different charges at physiological pH (e.g., nonpolar, uncharged but polar, negatively charged, positively charged); some groups are hydrophobic (e.g., branched chain and aromatic amino acids) and some hydrophilic (most others). These side chains have an important bearing on the ways in which the higher orders of protein structure are stabilized and are intimate parts of many other aspects of protein function.
How to treat a contaminated eye?
Perform CPR if necessary. Immediately flush contaminated eyes with gently flowing water. Do not induce vomiting. If vomiting occurs, lean patient forward or place on the left side (head-down position, if possible) to maintain an open airway and prevent aspiration. Keep patient quiet and maintain normal body temperature. Obtain medical attention. /Poisons A and B/
Is threonine an amino acid?
It is an aspartate family amino acid, a proteinogenic amino acid, a threonine and a L-alpha-amino acid. It is a conjugate base of a L-threoninium. It is a conjugate acid of a L-threoninate. It is an enantiomer of a D-threonine. It is a tautomer of a L-threonine zwitterion.
Threonine Definition
Threonine is an amino acid that we use in the biosynthesis of proteins. It involves an α-amino group (a protonated − NH3+ structure under organic conditions), a carboxyl group (a deprotonated − COO− form under biological conditions), and a side chain containing a hydroxyl group (OH), making it a polar, uncharged amino acid.
What is Threonine?
Threonine fills in as the sole source of carbon and nitrogen for the development of a wide assortment of creatures.
Biosynthesis of Threonine
From the above text, we understand that Threonine, a thr amino acid is obtainable from many proteins. It is among the several so-called essential amino acids. Animals cannot synthesize it and require dietary sources. Threonine is synthesized in microorganisms from the amino acid aspartic acid through α-aspartyl-semialdehyde and homoserine.
Threonine Structure
Threonine is among the two proteinogenic amino acids with two stereogenic centers, another being isoleucine.
Threonine Significance
L-threonine is an optically dynamic type of threonine having L-arrangement. It functions as the following:
L - Threonine Functions
Threonine is a fundamental amino acid, i.e., it is crucial for your wellbeing, however, it can’t be combined by your body and in this manner must be acquired from an eating routine. This amino acid backing focal apprehensive, cardiovascular, liver, and safe framework working - just to give some examples.
Threonine Role on Immune System
The body requires threonine for supporting appropriate safe capacities. It has been seen that the thymus organ uses this fundamental amino corrosive for animating the amalgamation of T - lymphocytes or White blood cells and assists with improving their movement.
How is threonine synthesized?
In plants and microorganisms, threonine is synthesized from aspartic acid via α-aspartyl-semialdehyde and homoserine. Homoserine undergoes O -phosphorylation; this phosphate ester undergoes hydrolysis concomitant with relocation of the OH group. Enzymes involved in a typical biosynthesis of threonine include:
When was threonine discovered?
Threonine was the last of the 20 common proteinogenic amino acids to be discovered. It was discovered in 1936 by William Cumming Rose, collaborating with Curtis Meyer. The amino acid was named threonine because it was similar in structure to threonic acid, a four-carbon monosaccharide with molecular formula C 4 H 8 O 5.
What are the most common small motifs formed by interactions with serine?
Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices).
Is threonine a protein?
Threonine is one of two proteinogenic amino acids with two stereogenic centers, the other being isoleucine. Threonine can exist in four possible stereoisomers with the following configurations: (2 S ,3 R ), (2 R ,3 S ), (2 S ,3 S) and (2 R ,3 R ). However, the name L -threonine is used for one single stereoisomer, ...
Is threonine a posttranslational modification?
The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can undergo O -linked glycosylation. In addition, threonine residues undergo phosphorylation through the action of a threonine kinase. In its phosphorylated form, it can be referred to as phosphothreonine. Phosphothreonine has three potential coordination sites (carboxyl, amine and phosphate group) and determination of the mode of coordination between phosphorylated ligands and metal ions occurring in an organism is important to explain the function of the phosphothreonine in biological processes.
What is threonine used for?
Threonine may interact with other drugs or agents used to treat Alzheimer’s disease, stomach disorders, stuffy nose, and high cholesterol conditions. It may also interact with pain relievers, medroxyprogesterone acetate ( a hormone for birth control) and NMDA antagonist ( a type of ).
What is the role of threonine in the body?
Threonine is an essential amino acid is vital for the normal function of your body . A deficiency of threonine, although rare, may lead to fatty liver, intestinal problems, and increased stress.
What amino acid is used to build lipotropic function?
Threonine works with other amino acids such as methionine and aspartic acid to build lipotropic function or digestion of fats. Without threonine, fats could accumulate in the liver, thereby causing fatty liver and thus liver failure.
How does threonine help with blood sugar?
Through gluconeogenesis ( the formation of new glucose), threonine proves useful in stabilizing blood sugar. The amino acid stabilizes the blood sugar level by maintaining the balance between glucose and insulin.
What is the function of intestinal mucin?
Intestinal mucin protects the intestinal wall from the digestive enzymes, toxin absorption into the body, bacteria clinging into the intestinal cells and intestinal water loss.
Can celiacs eat wheat?
Allows celiac patients to eat wheat. Celiac disease refers to an autoimmune disorder that causes gluten indigestion and finally small intestine damage. Patients who add 2000-4000 mg of threonine in their diets lead them to eat wheat without suffering celiac disease. (Healthifybody.com, 2019)
Does threonine help with Lou Gehrig's disease?
Research has shown that threonine may treat Lou Gehrig’s disease or Amyotrophic Lateral Sclerosis (ALS) that is a degenerative motor neuron disease. ALS characterizes symptoms such as tight muscles, trouble swallowing, chewing, nasal and slurred speech. In late stages, cognitive abilities remain intact but dementia can be possible. A double-blind study and open trials showed that threonine improved the ALS symptoms although some other research didn’t show some improvement. (6)
Types Of Amino Acids
The 20 amino acids that are used to make protein in the body can be classified in several ways, including their structure, electrical charge, polarity, and essentiality. We’re going to focus on essentiality, which looks at the amino acids in terms of the human body’s ability to produce them (or not).
Essential Amino Acids
These cannot be made by the body, and must therefore come from food sources. Some of the best sources of essential amino acids are meat, eggs, poultry, dairy products and dairy protein supplements.
Non-Essential Amino Acids
The human body has the ability to make these amino acids, even if we do not get it from the foods that we eat.
Conditionally Essential Amino Acids
These are amino acids that are not usually “essential,” but under conditions of illness or stress, they may become essential.
Types of amino acids
There are approximately twenty types of amino acids that combine with each other to form a huge group of different proteins. In general, amino acids are divided into three basic types. These types of amino acids include the following:
Functions of amino acids
Amino acids play an essential role in many body systems and their vital functions, and the functions of amino acids are as follows:
Food sources rich in essential amino acids
Although the body is not able to manufacture the essential amino acids, the body can get them from the daily diet in sufficient quantity, and there are many food sources rich in essential amino acids, especially food sources rich in proteins; Whether they are animal or vegetable sources, the following are some examples of these food sources:

Overview
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH 3 form under biological conditions), a carboxyl group (which is in the deprotonated −COO form under biological conditions), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essentialin humans, meani…
Modifications
The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can undergo O-linked glycosylation. In addition, threonine residues undergo phosphorylation through the action of a threonine kinase. In its phosphorylated form, it can be referred to as phosphothreonine. Phosphothreonine has three potential coordination sites (carboxyl, amine and phosphate group) and determination of the mode of coordination between …
History
Threonine was the last of the 20 common proteinogenic amino acids to be discovered. It was discovered in 1936 by William Cumming Rose, collaborating with Curtis Meyer. The amino acid was named threonine because it was similar in structure to threonic acid, a four-carbon monosaccharide with molecular formula C4H8O5
Stereoisomers
Threonine is one of two proteinogenic amino acids with two stereogenic centers, the other being isoleucine. Threonine can exist in four possible stereoisomers with the following configurations: (2S,3R), (2R,3S), (2S,3S) and (2R,3R). However, the name L-threonine is used for one single stereoisomer, (2S,3R)-2-amino-3-hydroxybutanoic acid. The second stereoisomer (2S,3S), which is rarely present in nature, is called L-allothreonine. The two stereoisomers (2R,3S)- and (2R,3R)-2-…
Biosynthesis
As an essential amino acid, threonine is not synthesized in humans, and needs to be present in proteins in the diet. Adult humans require about 20 mg/kg body weight/day. In plants and microorganisms, threonine is synthesized from aspartic acid via α-aspartyl-semialdehyde and homoserine. Homoserine undergoes O-phosphorylation; this phosphate esterundergoes hydrolysis conc…
Metabolism
Threonine is metabolized in at least three ways:
• In many animals it is converted to pyruvate via threonine dehydrogenase. An intermediate in this pathway can undergo thiolysis with CoA to produce acetyl-CoA and glycine.
• In humans the gene for threonine dehydrogenase is an inactive pseudogene, so threonine is converted to α-ketobutyrate. The mechanism of the first step is analogous to that catalyzed by serine dehydratase, and the se…
Sources
Foods high in threonine include cottage cheese, poultry, fish, meat, lentils, black turtle bean and sesame seeds.
Racemic threonine can be prepared from crotonic acid by alpha-functionalization using mercury(II) acetate.
External links
• Threonine biosynthesis
• CID 205
• CID 6288