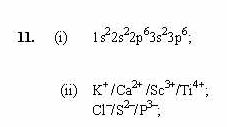What type of bond is formed between argon and gold atoms?
Molecules containing argon can be van der Waals molecules held together very weakly by London dispersion forces. Ionic molecules can be bound by charge induced dipole interactions. With gold atoms there can be some covalent interaction. Several boron-argon bonds with significant covalent interactions have been also reported.
Is argon ionic or covalent bond?
What type of bond does Argon form? If an element does not have full electron shells, it would either give or take electrons (ionic bonding) or share electrons (covalent bonding). Argon has an atomic number of 18 which means that it has 18 electrons.
What are argon compounds?
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space.
What is the binding energy of argon?
The argon binding energy is respectively 16.3, 1.01, 0.97 and 0.23 kcal/mol. The infrared absorption peak for Cu (CO) 3+ Ar is 2205 cm −1 compared to 2199 cm −1 for Cu (CO) 3+. For Cu (CO) 4+ Ar the peak is at 2198 cm −1 compared to 2193 for Cu (CO) 4+.
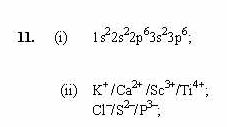
What type of bonding is in argon?
An Argon–Oxygen Covalent Bond in the ArOH+ Molecular Ion.
Is argon covalent or not?
The extremely stable noble gasses, including helium, neon, argon, krypton, xenon and radon, are all also nonmetal covalent elements. These elements form bonds with one another by sharing electrons to form compounds.
What is the ionic form of argon?
Positive argon ions (Ar+) are extracted from the plasma under the action of the electric field and bombard the target, which is at the cathode potential.
How does argon bond with itself?
It is very non-reactive. So much so, that it forms compounds with virtually no other elements. Just like neon (Ne) and helium (He), argon (Ar) usually floats around all by itself. It is non-reactive because the shells are full.
What elements form ionic bonds?
Ionic compounds generally form between elements that are metals and elements that are nonmetals. For example, the metal calcium (Ca) and the nonmetal chlorine (Cl) form the ionic compound calcium chloride (CaCl2).
Is argon polar or nonpolar?
nonpolarThe noble or inert gases are also considered nonpolar. These gases consist of single atoms of their element, such as argon, helium, krypton, and neon.
Why argon does not form ionic bonds or covalent bonds?
The Group 8A elements have a full octet of eight valence electrons in their highest-energy orbitals (ns2np6), so these elements have very little tendency to gain or lose electrons to form ions, or share electrons with other elements in covalent bonds.
What compounds does argon form?
Argon has been found to form one neutral compound with fluorine and hydrogen called argon fluorohydride (HArF). However, this compound is only stable at very cold temperatures (-256 degrees C). Where is argon found on Earth? Argon is the most abundant of the noble gases in the Earth's atmosphere.
What intermolecular forces are present in argon?
Molecules containing argon can be van der Waals molecules held together very weakly by London dispersion forces. Ionic molecules can be bound by charge induced dipole interactions.
How is the covalent bond formed?
A covalent bond consists of the mutual sharing of one or more pairs of electrons between two atoms. These electrons are simultaneously attracted by the two atomic nuclei. A covalent bond forms when the difference between the electronegativities of two atoms is too small for an electron transfer to occur to form ions.
Which of the following has covalent bond?
According to Fajan's rule small cation polarise anion upto greater extent , therefore AlCl3 has covalent bond between Al and Cl atom.
Does noble gases form covalent bond?
Because noble gases have full valency shell (8 electrons in outermost shell), which makes them chemically stable, and therefore have less tendency to lose or gain electrons. That's why they cannot form any bonds.
What is the ionic bond between argon and dinitrogen?
The argon ion can bond two molecules of dinitrogen (N 2) to yield an ionic complex with a linear shape and structure N=N− + Ar −N=N. The N=N bond length is 1.1014 Å, and the nitrogen to argon bond length is 2.3602 Å. 1.7 eV of energy is required to break this apart to N 2 and ArN+#N#2. The band origin of an infrared band due to antisymmetric vibration of the N=N bonds is at 2288.7272 cm −1. Compared to N 2 it is redshifted 41.99 cm −1. The ground state rotational constant of the molecule is 0.034 296 cm−1.
Why are argon compounds rare?
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space.
What is the name of the noble gas hydride?
Neutral argon hydride, also known as argon monohydride (ArH), was the first discovered noble gas hydride. J. W. C. Johns discovered an emission line of ArH at 767 nm and announced the find in 1970. The molecule was synthesized using X-ray irradiation of mixtures of argon with hydrogen-rich molecules such as H 2, H 2 O, CH 4 and CH 3 OH. The X-ray excited argon atoms are in the 4p state.
How does argon form van der Waals?
These can be made by expanding argon under high pressure mixed with the atoms of another element. The expansion happens through a tiny hole into a vacuum, and results in cooling to temperatures a few degrees above absolute zero. At higher temperatures the atoms will be too energetic to stay together by way of the weak London dispersion forces. The atoms that are to combine with argon can be produced by evaporation with a laser or alternatively by an electric discharge. The known molecules include AgAr, Ag 2 Ar, NaAr, KAr, MgAr, CaAr, SrAr, ZnAr, CdAr, HgAr, SiAr, InAr, CAr, GeAr, SnAr, and BAr. SiAr was made from silicon atoms derived from Si (CH 3) 4.
What wavelength is the argon fluoride laser?
The widely used argon fluoride laser makes use of the ArF* excimer to produce strong ultraviolet radiation at 192 nm. The argon chloride laser using ArCl* produces even shorter ultraviolet at 175 nm, but is too feeble for application. The argon chloride in this laser comes from argon and chlorine molecules.
How is ArbeO formed?
When beryllium atoms react with oxygen in a solid argon matrix (or beryllia is evaporated into the matrix) ArBeO will be formed, and is observable by its infrared spectrum. The beryllia molecule is strongly polarised, and the argon atom is attracted to the beryllium atom. The bond strength of Ar−Be is calculated to be 6.7 kcal/mol (28 kJ/mol). The Ar−Be bond length is predicted to be 2.042 Å.
What is the ionic ion of Ar 1 C 60?
Ar 1 C 60 was discovered by the CSIRO . Argon ionises at 15.76 eV, which is higher than hydrogen, but lower than helium, neon or fluorine . Molecules containing argon can be van der Waals molecules held together very weakly by London dispersion forces. Ionic molecules can be bound by charge induced dipole interactions.