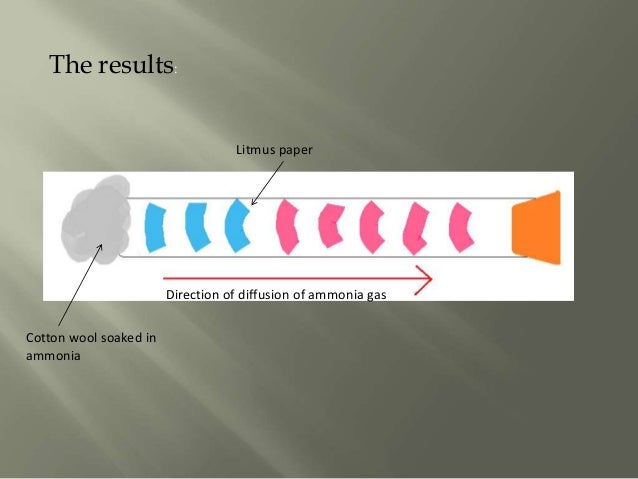
Why is ammonia harmful to humans?
☞ Once inhaled, ammonia immediately interacts with moisture in the mucus to form caustic ammonium hydroxide. As a result, inhalation of ammonia vapors may cause irritation of the eyes, nose, skin, throat, and respiratory tract. ☞ Low concentrations of ammonia may aggravate the respiratory condition of asthma patients.
What are facts about ammonia?
- The production of ammonia is used by more than 1% of total global energy
- Never combine ammonia with bleach as this creates a poisonous gas
- Many fertilizers and household cleaning products contain ammonia
- Some household cleaning products can contain up to 10% ammonia
Why is NH3 a base?
Why is nh3 base? Ammonia is considered as a base because Nitrogen atom in it contains lone pair of electrons hence it can donate electrons to other atoms therefore it can be considered as base . Click to see full answer .
How to write the chemical formula for ammonia gas?
Ammonia’s molecular formula is NH3. Its molar mass is 17.0306g. Its appearance is a colorless gas. The molecular shape is a Terminus. The bond angle is 107.5. Ammonia is a chemical compund with the formula NH3. Usually, it's a colorless gas with a pungent odor and is seen in some houshold cleaning solutions.
See more
Is ammonia gas a basic gas?
Ammonia is moderately basic; a 1.0 M aqueous solution has a pH of 11.6, and if a strong acid is added to such a solution until the solution is neutral (pH = 7), 99.4% of the ammonia molecules are protonated.
What kind of gas is in ammonia?
The New York Department of Health describes ammonia gas as a colorless, alkaline gas comprised of nitrogen and hydrogen (NH3) that has a strong odor, often associated with window cleaner. Ammonia is a natural, biological agent in organisms that helps to form amino acids, which are the basic building blocks of proteins.
Is ammonia a toxic gas?
Ammonia is a toxic gas or liquid that, when concentrated, is corrosive to tissues upon contact. Exposure to ammonia in sufficient quantities can be fatal. One of the highest production-volume chemicals in the U.S., concentrated ammonia is used in manufacturing, refrigeration, and agriculture (as a fertilizer).
Can you burn ammonia as fuel?
Ammonia can be used as a fuel but there are several challenges in ammonia combustion, such as low flammability, high NOx emission, and low radiation intensity. Overcoming these challenges requires further research into ammonia flame dynamics and chemistry.
What is ammonia gas used for?
Ammonia is a naturally occuring gas that serves as a chemical building block for a range of commercial and household products, including fertilizers and cleaning supplies. Additional uses include as a refrigerant, stabilizer, neutralizer, and purifier — particularly in food transport and water treatment applications.
Where does ammonia gas come from?
Ammonia is a naturally occurring compound made up of a single nitrogen atom and three hydrogen atoms. Mostly found in trace quantities, it's a fundamental part of the natural nitrogen cycle. The breakdown of organic waste matter is a key source of naturally produced ammonia, making livestock farms a major source.
What is ammonia gas?
The New York Department of Health describes ammonia gas as a colorless, alkaline gas comprised of nitrogen and hydrogen (NH3) that has a strong odor, often associated with window cleaner. Ammonia is a natural, biological agent in organisms that helps to form amino acids, which are the basic building blocks of proteins. It also involved in the natural decomposition of plant and animal materials. When present in higher concentrations, ammonia gas is hazardous to workers and the public.
What temperature does ammonia have?
According to the National Institute for Occupational Safety and Health, ammonia has boiling temperature of -33°C (-28°F) — ammonia is a gas a room temperature. While it is lighter than air, a release of pressurized ammonia gas can collect at ground-level until the aerosol cloud becomes diluted and begins to rise.
How much ammonia is safe to work with?
In the United Kingdom, the Health and Safety Executive specifies safe exposure limits for ammonia gas as 25 ppm for an eight-hour time-weighted average (TWA) and 35 ppm for a 15-minute short-term exposure limit (STEL).
What is a G7 gas sensor?
G7 features an ammonia gas sensor option that alerts live monitoring personnel when an employee needs help, directing responders to the employee's exact location. Best of all, G7 safety monitoring technology and cloud-based monitoring portal are a turnkey solution that don't rely on facility Wi-Fi networks or power to operate. As a standalone system, no smartphones or Bluetooth connections are required.
How much ammonia can cause irritation?
According to Alberta Agriculture and Forestry, nose and throat irritation can result from ammonia exposure ranging from 24–50 parts per million (ppm) after ten minutes of exposure. With a higher ammonia concentration ranging from 72–134 ppm, the same irritation can occur in half the time. For a concentration of 700 ppm, immediate and severe irritation would likely occur. At a concentration of 5,000 ppm, respiratory spasms and rapid suffocation occurs. At 10,000 ppm, pulmonary edema and potentially fatal accumulation of liquid in the lungs would occur.
What is the maximum exposure limit for ammonia?
In the United States, OSHA specifies the permissible exposure limit (PEL) for ammonia gas as 50 ppm for an eight-hour TWA, allowing a higher limit of 100 ppm for a shorter four-hour TWA.
How much ammonia does a person smell?
Although not every organization agrees on the perceptible threshold, OSHA estimates that people begins to smell ammonia gas ranging from 5 to 50 ppm. Experience in the industry indicates that workers consistently subjected to weak ammonia levels may become somewhat desensitized to its presence.
What is the color of ammonia?
Liquid ammonia is used extensively as a nonaqueous solvent. The alkali metals as well as the heavier alkaline-earth metals and even some inner transition metals dissolve in liquid ammonia, producing blue solutions. Physical measurements, including electrical-conductivity studies, provide evidence that this blue colour and electrical current are due to the solvated electron. metal (dispersed) ⇌ metal (NH 3) x ⇌ M + (NH 3) x + e− (NH 3) y These solutions are excellent sources of electrons for reducing other chemical species. As the concentration of dissolved metal increases, the solution becomes a deeper blue in colour and finally changes to a copper-coloured solution with a metallic lustre. The electrical conductivity decreases, and there is evidence that the solvated electrons associate to form electron pairs. 2 e− (NH 3) y ⇌ e2 (NH 3) y Most ammonium salts also readily dissolve in liquid ammonia.
What is the boiling point of ammonia?
Ammonia is a colourless gas with a sharp, penetrating odour. Its boiling point is −33.35 °C (−28.03 °F), and its freezing point is −77.7 °C (−107.8 °F). It has a high heat of vaporization (23.3 kilojoules per mole at its boiling point) and can be handled as a liquid in thermally insulated containers in the laboratory.
What is the reaction of ammonia to oxygen?
4NH 3 + 3O 2 + heat → 2N 2 + 6H 2 O However, with the use of a catalyst and under the correct conditions of temperature, ammonia reacts with oxygen to produce nitric oxide, NO, which is oxid ized to nitrogen dioxide, NO 2 , and is used in the industrial synthesis of nitric acid.
What is the process of converting ammonia into nitric acid?
Ammonia also finds application in both the ammonia-soda process (also called the Solvay process), a widely used method for producing soda ash, and the Ostwald process, a method for converting ammonia into nitric acid. Ammonia is used in various metallurgical processes, including the nitriding of alloy sheets to harden their surfaces.
What is the simplest stable compound of nitrogen and hydrogen?
Ammonia (NH3), colourless, pungent gas composed of nitrogen and hydrogen. It is the simplest stable compound of these elements and serves as a starting material for the production of many commercially important nitrogen compounds.
What is the most commonly used source of nitrogen for fertilizer worldwide?
Urea, (H 2 N) 2 C=O, is the most commonly used source of nitrogen for fertilizer worldwide. Ammonia is also used in the manufacture of commercial explosives (e.g., trinitrotoluene [TNT], nitroglycerin, and nitrocellulose ).
Where is ammonia applied?
In the United States, it is usually applied directly to the soil from tanks containing the liquefied gas. The ammonia can also be in the form of ammonium salts, such as ammonium nitrate, NH 4 NO 3, ammonium sulfate, (NH 4) 2 SO 4, and various ammonium phosphates.
Where does ammonia come from?
Ammonia occurs naturally and is produced by human activity. It is an important source of nitrogenwhich is needed by plants and animals. Bacteria found in the intestines can produce ammonia. Ammonia is a colorless gas with a very distinct odor.
How is ammonia produced?
Ammonia occurs naturally and is produced by human activity . It is an important source of nitrogen which is needed by plants and animals. Bacteria found in the intestines can produce ammonia. Ammonia is a colorless gas with a very distinct odor. This odor is familiar to many people because ammonia is used in smelling salts, many household and industrial cleaners, and window-cleaning products. Ammonia gas can be dissolved in water. This kind of ammonia is called liquid ammonia or aqueous ammonia. Once exposed to open air, liquid ammonia quickly turns into a gas. Ammonia is applied directly into soil on farm fields, and is used to make fertilizers for farm crops, lawns, and plants. Many household and industrial cleaners contain ammonia.
How is ammonia lost from water?
Ammonia is lost from water by volatilization (1). The Henry's Law constant for ammonia has been measured as 1.61X10-5 atm-cu m/mole (2). This Henry's Law constant indicates that ammonia is expected to volatilize from water surfaces (3). Based on this Henry's Law constant, the volatilization half-life from a model river (1 m deep, flowing 1 m/sec, wind velocity of 3 m/sec) (3) is estimated as 1.4 days (SRC). The volatilization half-life from a model lake (1 m deep, flowing 0.05 m/sec, wind velocity of 0.5 m/sec) (3) is estimated as 12 days (SRC). Ammonia's Henry's Law constant indicates that volatilization from moist soil surfaces may occur (SRC). Ammonia is a gas with a vapor pressure of 7500 mm Hg at 25 °C and atmospheric pressure (4), and therefore, is expected to volatilize from dry soil surfaces (SRC).
How is ammonia converted to nitrate?
AEROBIC: When ammonia appears in water under the normal conditions (aerobic), it is rapidly converted to nitrate by nitrification; the principal water contaminant normally being nitrate. The pH in water is increased by the presence of ammonia ion, in the form of hydroxide ions. ... Bacteria convert the ammonia to nitrate creating an oxygen demand (BOD) several days after the introduction of ammonia. The bacteria that oxidize ammonia to nitrate are largely of the genus Nitrosomonas; conversion of nitrite to nitrate is carried out primarily by the genus Nitrobacter. Temperature, oxygen supply, and pH of the water are factors in determining the rate of oxidation.
What causes ammonia to react with acids?
Ammonia solutions react exothermically with acids to produce water and ammonium salts, Heating or treating with strong bases also causes evolution of gaseous ammonia. Ammonia can burn or explode if exposed to an intense source of ignition but can generally be treated as nonflammable. Readily combines with silver oxide, silver chloride, silver nitrate, silver azide or mercury to form explosive compounds. Forms explosive ammonium chlorate on contact with chlorates [Kirk-Othmer, 3rd ed., Vol. 2, 1978, p. 470]. Reacts violently or produces explosive products with fluorine, chlorine, bromine and iodine and bromine pentafluoride and chlorine trifluoride. Mixing of bleaching powder ( hypochlorite solution) with ammonia solutions produces toxic/explosive ammonia trichloride vapors. May react violently with boron halides, ethylene oxide (polymerization), perchlorates and strong oxidizing agents ( chromyl chloride, chromium trioxide, chromic acid, nitric acid, hydrogen peroxide, chlorates, fluorine, nitrogen oxide, liquid oxygen ).
What is ammonia used for?
Ammonia in solution ... in varying concentration is used in variety of products such as cleaning agents, liniments, and aromatic spirits. Ammonia solution are sometimes used as fertilizers ... Fresh household ammonia ranges in concentration from 5 to 10% NH3, but a 54% solution is also available commercially.
How much ammonia is produced in the human body?
Human adults produce around 1000 mmol of ammonia daily. Some is reutilized in biosynthesis. The remainder is waste and neurotoxic. Eventually most is excreted in urine as urea, together with ammonia used as a buffer. In extrahepatic tissues, ammonia is incorporated into nontoxic glutamine and released into blood. Large amounts are metabolized by the kidneys and small intestine. In the intestine, this yields ammonia, which is sequestered in portal blood and transported to the liver for ureagenesis, and citrulline, which is converted to arginine by the kidneys. The amazing developments in NMR imaging and spectroscopy and molecular biology have confirmed concepts derived from early studies in animals and cell cultures. The processes involved are exquisitely tuned. When they are faulty, ammonia accumulates. Severe acute hyperammonemia causes a rapidly progressive, often fatal, encephalopathy with brain edema. Chronic milder hyperammonemia causes a neuropsychiatric illness. Survivors of severe neonatal hyperammonemia have structural brain damage. Proposed explanations for brain edema are an increase in astrocyte osmolality, generally attributed to glutamine accumulation, and cytotoxic oxidative/nitrosative damage. However, ammonia neurotoxicity is multifactorial, with disturbances also in neurotransmitters, energy production, anaplerosis, cerebral blood flow, potassium, and sodium. Around 90% of hyperammonemic patients have liver disease. Inherited defects are rare. They are being recognized increasingly in adults. Deficiencies of urea cycle enzymes, citrin, and pyruvate carboxylase demonstrate the roles of isolated pathways in ammonia metabolism. Phenylbutyrate is used routinely to treat inherited urea cycle disorders, and its use for hepatic encephalopathy is under investigation. /Hyperammonemia/
Why is ammonia in water so high?
Slow-moving water: Slow-moving or stagnant water (see Figure 5) may have high ammonia concentrations because of lack of turbulence and volatilization and greater accumulation of metabolic waste and decomposition products —including ammonia (WHO 1986).
How is ammonia affected by human activities?
The more extensive the relevant sources and activities, the more likely it is that ammonia will reach concentrations that can impair surface waters.
What causes higher ammonia concentrations in the hypolimnion?
Lack of turbulence and mixing will decrease volatilization of ammonia, resulting in higher ammonia concentrations downstream. Thermal stratification in impoundments can lead to higher concentrations of ammonia in the hypolimnion which, with bottom-release dams, can result in increased ammonia in downstream waters.
What causes ammonia in sediment?
Ammonia in sediments typically results from bacterial decomposition of organic matter that accumulates in sediment. Sediment microbiota mineralize organic nitrogen or (less commonly) produce ammonia by dissimilatory nitrate reduction. Ammonia is especially prevalent in anoxic sediments because nitrification (the oxidation of ammonia to nitrite [NO 2-] and nitrate [NO 3- ]) is inhibited. Ammonia generated in sediment may be toxic to benthic or surface water biota (Lapota et al. 2000).
How does high algal production affect ammonia?
High plant production: High algal or plant production can decrease ammonia by assimilation, increase ammonia by nitrogen fixation, or increase pH toxicity by CO 2 uptake, resulting in a shift to more unionized ammonia. Top of Page.
What is the cause of ammonia in septic systems?
Septic seepage and failed package plants: Seepage from failed septic tanks or their leach fields, and discharges from poorly–functioning package sewage treatment plants may contribute significant amounts of ammonia to streams and lakes.
How does channel alteration affect ammonia?
Channel alteration can result in decreased nitrogen uptake within the stream, while decreases in riparian and watershed vegetation associated with agriculture and urbanization can reduce nitrogen uptake in the surrounding landscape. Channel alteration and water withdrawals can reduce ammonia volatilization due to changes in water velocities and depths.
Where is ammonia found?
Ammonia (NH3) is found throughout the environment in the air, soil, and water, and in plants and animals, including humans. Ammonia is also found in many household and industrial cleaners. High levels of ammonia can irritate and burn the skin, mouth, throat, lungs, and eyes. Very high levels of ammonia can damage the lungs or cause death.
What are the hazards of ammonia?
Ammonia is used in many industries. Some examples of workers at risk of being exposed to ammonia include the following: 1 Agricultural workers who use soil fertilizer 2 Industrial workers who manufacture fertilizers, rubber, nitric acid, urea, plastics, fibers, synthetic resin, solvents and other chemicals 3 Miners and metallurgic workers 4 Workers in petroleum refining 5 Workers who use a commercial refrigerant in food processing, produce ice, are near cold storage and de-icing operations.
What are some examples of workers exposed to ammonia?
Ammonia is used in many industries. Some examples of workers at risk of being exposed to ammonia include the following: Agricultural workers who use soil fertilizer.
What is the NIOSH guidelines for chemical hazards?
NIOSH Guidelines for Chemical Hazards DHHS Publication No. 74-136 (1974) – Contains a standard for mitigation of exposure to ammonia to prevent adverse effects over a working lifetime.
What are the byproducts of ammonia?
Byproducts. One of the main industrial byproducts of ammonia production is CO 2. In 2018, high oil prices resulted in an extended summer shutdown of European ammonia factories causing a commercial CO 2 shortage, thus limiting production of carbonated drinks such as beer and fizzy soft drinks.
What is the most ammonia produced in the world?
China produced 31.9% of the worldwide production, followed by Russia with 8.7%, India with 7.5%, and the United States with 7.1%. 80% or more of the ammonia produced is used for fertilizing agricultural crops. Ammonia is also used for the production of plastics, fibers, explosives, nitric acid ...
How is hydrogen made from hydrocarbons?
The method for producing hydrogen from hydrocarbons is known as steam reforming. The hydrogen is then combined with nitrogen to produce ammonia via the Haber-Bosch process . The first step in the process is to remove sulfur compounds from the feedstock because sulfur deactivates the catalysts used in subsequent steps.
What is the process of synthesis of ammonia?
To produce the desired end-product ammonia, the hydrogen is then catalytically reacted with nitrogen (derived from process air) to form anhydrous liquid ammonia. This step is known as the ammonia synthesis loop (also referred to as the Haber-Bosch process):
How much hydrogen gas was produced in Iceland in 2002?
For example, in 2002, Iceland produced 2,000 tons of hydrogen gas by electrolysis, using excess electricity production from its hydroelectric plants, primarily for the production of ammonia for fertilizer.
How to recover ammonia from water?
Another option for recovering ammonia from waste water is to use the mechanics of the ammonia-water thermal absorption cycle. Using this option, ammonia can be recovered either as a liquid or as ammonium hydroxide. The advantage of the former is that it is much easier to handle and transport, whereas the latter also has a commercial value when a concentration of 30 percent ammonium hydroxide in solution is produced.
What is the catalyst used in ammonia synthesis?
Due to the nature of the (typically multi-promoted magnetite) catalyst used in the ammonia synthesis reaction, only very low levels of oxygen-containing (especially CO, CO 2 and H 2 O) compounds can be tolerated in the synthesis (hydrogen and nitrogen mixture) gas.
What is the main use of ammonia?
The main use of ammonia is agricultural (fertilizer). More than 80 percent of the ammonia produced is utilized in this fashion due to its high nitrogen content. Since ammonia is plentiful, the cost is low.
Why Ammonia?
The main use of ammonia is agricultural (fertilizer). More than 80 percent of the ammonia produced is utilized in this fashion due to its high nitrogen content . Since ammonia is plentiful, the cost is low. The cost for refrigerant-grade anhydrous ammonia typically is less than 50 cents a pound, compared to about $7 a pound for R4O4A and $15 a pound for R-502.
What is the minimum water content for ammonia?
There is an agricultural or commercial-grade ammonia that must contain a minimum water content of at least 2,000 ppm water (0.2 percent) with a maximum water content of 5,000 ppm (0.5 percent).
What is industrial grade ammonia?
Industrial-grade anhydrous ammonia, commonly called metallurgical or refrigeration grade, has very little water contamination. Metallurgical-grade has a maximum of about 33 ppm water (0.0033 percent) and refrigeration-grade has a maximum of about 150 ppm water (0.015 percent). For optimum efficiency and effectiveness in your refrigeration system, ...
Can ammonia leaks be accidents?
Most serious cases of leaks and/or explosions can be attributed to accidents. (The U.S. Occupational Safety and Health Administration (OSHA) and the International Institute of Ammonia Refrigeration (AR) do not keep injury and fatality statistics for ammonia-based systems.)
Is ammonia the oldest refrigerant?
As far back as we can remember man has used ammonia for one reason or another. It is in fact the oldest known refrigerant. It is successfully utilized in many industries including food, petrochemical and pharmaceutical. So why is it so misunderstood?
Is ammonia void of water?
In this and the next issue of RSES Journal we will attempt to clear up and demystify anhydrous ammonia, which is a formulation void of water, as it relates to refrigeration. We will discuss the benefits and drawbacks of this efficient refrigerant as well as review leak detection and safe handling procedures.
What is gas in science?
Definition of Gas. Gas is a matter whose molecules are bound by week forces of attraction such that they are widely separated from each other. Due to this, they are invisible and also without a specific shape. Since any form of volatile matter is called a gas, there are many others as well besides above.
How are gases formed?
Chemically, these are combinations of two or more elements. They are formed by the combination of carbon, oxygen, nitrogen, hydrogen, and sulfur. Ex: carbon-dioxide (CO2), methane (CH4), sulfur dioxide (SO2), ammonia (NH3), etc. Natural gases are present even before life existed on ...
What is nitrogen used for?
Nitrogen is an important element used as fertilizer . It is converted from air into ammonia and fixed in the soil by some bacteria. This ammonia acts as a fertilizer to the crop. When the crop is destroyed, the gas is again returned back to the atmosphere.
What gases do you come across during your first science class?
The gases you come across during your initial science classes are natural.
How do gases convert to gas?
These gases take the heat from within and release it out by condensing to liquid. They then convert to gas by taking the internal heat. Thus they convert from the liquid phase to gas phases alternatively. This way, they can aid in cooling the compartments in a fridge and also in air conditioners.
What is the purpose of gas chromatography?
Materials used in drug manufacture, nutrition, and research are analyzed. In gas chromatography, nitrogen is used as a carrier gas.
What is the color of natural gas?
As fuel for cooking and other areas. For domestic cooking, natural gas is widely used. The gas produces a blue color flame of high temperature. Besides, this flame also does not form a black suit and smoke, unlike traditional fuels. Even for automobiles, there is research going on for the use of hydrogen as fuel.
.png)