
Can humans break down the glycosidic bonds in cellulose?
Wood and paper are mostly made of cellulose, and cellulose itself is made up of unbranched chains of glucose monomers linked by 1 4 glycosidic bonds. The β glycosidic linkages in cellulose can't be broken by human digestive enzymes, so humans are not able to digest cellulose. Can humans metabolize cellulose?
What is meant by glycosidic linkage?
What do you understand by the term glycosidic linkage? Glycosidic linkage refers to the linkage formed between two monosaccharide units through an oxygen atom by the loss of a water molecule. For example, in a sucrose molecule, two monosaccharide units, ∝-glucose and β-fructose, are joined together by a glycosidic linkage.
What is an example of a glycosidic linkage?
What do you understand by the term glycosidic linkage?
- Answer
- Glycosidic linkage refers to the linkage formed between two monosaccharide units through an oxygen atom by the loss of a water molecule.
- For example, in a sucrose molecule, two monosaccharide units, ∝-glucose and β-fructose, are joined together by a glycosidic linkage.
What is the glycosidic linkage formula?
They have the same chemical formula, C12H22O11, but their structures are different.
See more
What type of glycosidic linkage is cellulose?
beta-All the glucose molecules in cellulose have the beta-configuration at the C1 atom, so all the glycosidic bonds that join the glucose molecules together are also of the beta type. This means that the cellulose molecule is straight, and many such molecules can lay side by side in a parallel series of rows.
Which glycosidic linkage is present in cellulose find from the following?
Cellulose is linear homopolysaccharide of D-glucose units, which are linked together by beta-1,4 glycosidic bonds. This makes option D correct answer.
What type of glycosidic linkage can be found in cellulose quizlet?
Cellulose consists of beta-glucose monomers joined by beta-1,4-glycosidic linkages.
What is the linkage between cellulose?
Cellulose is a linear polymer glucan and is composed of glucose units (> 10 000), which are linked by β-(1–4)-glycosidic bonds.
Which has beta glycosidic linkage?
beta - glycosidic linkage is present in maltose.
What is an alpha 1 4 glycosidic linkage?
When two alpha D-glucose molecules join together a more commonly occurring isomer of glucose compared to the L-glucose, form a glycosidic linkage, the term is known as a α-1,4-glycosidic bond.
What type of bond is found in cellulose quizlet?
What is the structure of cellulose? A polysaccharide consisting of beta-glucose monomers joined by beta-1,4 glycosidic linkages. Each glucose molecule is flipped in relation to the ones beside it. There are hydrogen bonds between parallel strands.
What is a 1/6 glycosidic bond?
From The School of Biomedical Sciences Wiki. An α-1,6-glycosidic bond is a covalent bond formed between the -OH group on carbon 1 of one sugar and the -OH group on carbon 6 of another sugar. This linkage causes branching within the polyscaccharide.
What type of glycosidic linkage is between the two monosaccharides?
Disaccharides are composed of two monosaccharide units linked together by a glycosidic bond. The most common glycosidic bonds connecting monosaccharide units are O-glycosidic bonds in which the oxygen from a hydroxyl group becomes linked to the carbonyl carbon.
What is N glycosidic linkage?
A glycosidic bond or glycosidic linkage is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.
How do you identify alpha and beta glycosidic linkage?
1:402:34What is a Glycosidic bond? Difference between alpha and beta ... - YouTubeYouTubeStart of suggested clipEnd of suggested clipIn short in alpha 1-4 glycosidic linkage. The Oh H group in the first anomeric carbon is below theMoreIn short in alpha 1-4 glycosidic linkage. The Oh H group in the first anomeric carbon is below the plane whereas in beta 1-4 glycosidic linkage.
Does cellulose have alpha linkages?
Starches like amylose and amylopectin link only alpha-type glucose molecules together. With cellulose, it is the beta molecules that link together.
Which of the following glycosidic linkage is found in?
glycosidic linkage is found in maltose.
What are the glycosidic linkages found in starch and cellulose?
Starch contains glucose residues as α(1-4) glycosidic bonds in amylose, while glycosidic bonds at branching points in amylopectin α(1-6), otherwise α(1-4) bonds. Cellulose constitutes their residues of glucose as glycosidic bonds with β(1-4).
Which of the following has no glycosidic linkage 1 sucrose 2 maltose 3 lactose 4 galactose?
The glucose and fructose monomers do not have any glycosidic bonds in them. Maltose is a disaccharide made of two glucose units and an alpha 1,4 glycosidic bond is seen. Sucrose is a disaccharide made up of glucose and fructose with both alpha and beta linkages. Hence, the correct answer is option C.
Which of the following is a glycosidic bond?
So, the correct answer is ' Polysaccharide and Water'.
What is the distribution of glycosidic linkages?
The distribution of glycosidic linkages and the relative amount of cello-oligomers in the β-glucan chain can be construed from the enzymatic hydrolysis of these polymers with (1 → 3) (1 → 4)-β- d -glucan-4-glucanohydrolase (EC 3.2.1.73). The enzyme, commonly known as lichenase, specifically cleaves the (1 → 4) linkage of the 3- O -substituted glucose unit in the β-glucan chain. The hydrolysis generates mostly oligosaccharides with degrees of polymerization (DPs) of three and four – 3- O -β-cellobiosyl- d -glucose (DP3) and 3- O -β-cellotriosyl- d -glucose (DP4) – which originate from the cellotriose and cellotetraose units in the polymeric chain ( Figure 1 ). Longer celluloselike fragments with DP ≥ 5 are also generated during the enzymatic hydrolysis, as shown in Figure 1. Several techniques have been used to analyze the oligosaccharides from lichenase-hydrolyzed β-glucans, including high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD), reverse-phase chromatography, capillary electrophoresis, and MS. HPAEC-PAD was first used by Wood et al. to characterize oligosaccharides from lichenase-hydrolyzed barley β-glucans and became the most frequently used technique for revealing the molecular structure of cereal β-glucans. Proper quantification of oligosaccharides by HPAEC-PAD requires the knowledge of weight response factors for each oligomer and is complicated by the fact that the sensitivity of PAD may decrease with increasing DP of oligosaccharides. Typical HPAEC profile of oligosaccharide released from lichenase-digested β-glucans previously extracted with water at 65 °C from barley endosperm cell walls is shown in Figure 3. The two most abundant oligomers are 3- O -β-cellobiosyl- d -glucose (DP3) and 3- O -β-cellotriosyl- d -glucose (DP4), representing about 88–92% of soluble oligosaccharides present in the digest. The abundance of oligomers with DP 5–11 decreases with the increasing DP, except for DP9 oligomer that shows atypical abundance compared to other cello-oligomers. Cello-oligosaccharides with DP > 11 are also released during lichenase digestion of β-glucans; however, they are more difficult to detect and quantify because of their lower abundance and their tendency to aggregate and precipitate out of solution once released from the polymer chains. The insoluble precipitate generated during enzymatic hydrolysis of β-glucans can be partially dissolved in solvents capable of breaking H-bonds, such as dimethyl sulfoxide, and their analysis by HPAEC-PAD reveals the presence of cello-oligomers with DP up to 28 and again shows the preponderance of DP9 ( Figure 4 ). Specific fractions of β-glucans extracted with water from barley and subsequently hydrolyzed with lichenase were shown to yield between 3% and 5% of these insoluble cello-oligosaccharides (based on the amount of the unhydrolyzed β-glucans).
What are the sources of polysaccharides?
Natural resource with rich polysaccharides are mainly from plant, fungus, and marine organism. Chinese herb medicine is a conventional plant source for bioactive polysaccharides, such as Dendrobium plants, Angelica sinensis, A. membranaceus, Bupleurum plants, Jujube fruit, and Aloe vera. Pectin, chitin, chitosan, guar and other more complicated polysaccharide extracted from medicine plants have been reported to have high antioxidant activity. Fungal polysaccharides are famous as its antioxidant function, which make it possible for food therapy. The source of novel bioactive compounds from marine organism has been concerned recently. The cell walls of marine algae, red algae, brown algae, and green algae are rich in sulfated polysaccharides such as fucoidans, carrageenans, and ulvans. These natural polysaccharides from algae are reported to be made into medicines with the outstanding antioxidant activity to prevent potential health risks like cardiovascular disease and cancer.
What are the determinants of a glucan chain?
The ratio of DP3/DP4 and the presence of celluloselike fragments in β-glucan chains are important determinants of their solubility, extractability, and rheological properties . Two possible mechanisms for intermolecular associations between β-glucan chains have been proposed. One involves the side-by-side associations of celluloselike segments of more than three contiguous β- (1 → 4)-linked glucosyl units, leading to the formation of aggregates, and the other refers to the interactions of chain segments with consecutive cellotriosyl units linked by β- (1 → 3) bonds that might form extended junction zones. The latter has been supported by findings that a helix made up of at least three consecutive cellotriosyl residues could constitute a stable ordered motif in β-glucan chains, as shown by x-ray analysis, and that the β- (1 → 3) linkages could be directly involved in the ordered conformation of barley β-glucans, as revealed by CP/MAS 13 C-NMR spectroscopy. Lazaridou et al. showed that an increasing DP3/DP4 ratio increases the tendency of cereal β-glucans to form aggregates and enhances their gelation potential. Moreover, high ratios of DP3/DP4 and large amounts of long celluloselike fragments (DP ≥ 5) in β-glucan chains have been associated with decreased solubility or extractability of these polysaccharides from cereal grains.
What are the two main polysaccharides in roots?
Polysaccharide is polymerized carbohydrate molecules consisting of long chains of monosaccharides combined by glycosidic linkages. Starch and cellulose are two important polysaccharides in roots and tubers. Both starch and cellulose are insoluble in water. As a storage polysaccharide, starch is a glucose polymer where hundreds of glucose units are connected by alpha-linkages (Coppin et al., 2005 ). Cellulose is structural polysaccharide made with numerous glucose units bonded using beta-linkages ( Lawther, Sun, & Banks, 1995 ). Predictions of starch and cellulose in roots and tubers are of interest. For instance, starch can be used to evaluate crop potential and the efficiency of processing enzymes which are used to convert starch into more valuable products for industrial applications.
How to analyze lichenase?
The products of lichenase digestion can also be analyzed by fluorophore-assisted capillary electrophoresis (FACE). The reducing ends of the liberated oligosaccharide chains are derivatized with the fluorescent dye (8-amino-1,3,6-pyrenetrisulfonic acid), separated by capillary electrophoresis, and monitored with a laser-induced fluorescence detector. Figure 5 shows an electropherogram obtained from capillary electrophoresis separation of lichenase-hydrolyzed oat β-glucan with well-resolved oligomers with DP up to 10. A key advantage of the reductive amination technique is that a single fluorophore is attached per reducing end and the detector response remains constant as the oligomeric chain length increases, thus enabling a molar quantification. The molar ratios of DP3/DP4 ranging from 1.6 to 1.7 were obtained for three different oat lines analyzed by this method; these values were slightly lower than values of 2.1–2.4 reported by Wood et al., who obtained their results by HPAEC-PAD and with no correction for differences in response factors.
What are the functions of amylases?
Amylases are crucial enzymes which hydrolyze internal glycosidic linkages in starch and produce as primary products dextrins and oligosaccharides. Amylases are classified into α-amylase, β-amylase, and glucoamylase based on their three-dimensional structures, reaction mechanisms, and amino acid sequences. Amylases have innumerable applications in clinical, medical, and analytical chemistries as well as in food, detergent, textile, brewing, and distilling industries. Amylases can be produced from plants, animals, and microbial sources. Due to the advantages in microbial production, it meets commercial needs. The pervasive nature, easy production, and wide range of applications make amylase an industrially pivotal enzyme. This chapter will focus on amylases found in marine microorganisms, their potential industrial applications, and how these enzymes can be improved to the required bioprocessing conditions.
Why do stiff chains have high viscosity?
Once dissolved, stiff chains lead to high viscosities because each polymer sweeps out a large volume of solvent. This ‘hydrodynamic volume’ is typically encapsulated by the measure of intrinsic viscosity (dL/g). Many rheological properties of polysaccharide solutions scale to the intrinsic viscosity ( Morris et al., 1981 ).
Where is cellulose obtained?
Cellulose for industrial use is mainly obtained from wood pulp and cotton. Some animals, particularly ruminants and termites, can digest cellulose with the help of symbiotic micro-organisms that live in their guts, such as Trichonympha.
How is cellulose broken down?
Cellulose is derived from D -glucose units, which condense through β (1→4)- glycosidic bonds. This linkage motif contrasts with that for α (1→4)-glycosidic bonds present in starch and glycogen.
What is a molecule that is soluble in water?
Molecules with very small chain length resulting from the breakdown of cellulose are known as cellodextrins ; in contrast to long-chain cellulose, cellodextrins are typically soluble in water and organic solvents.
How is cellulose treated?
Cellulose pulp may also be treated with strong acid to hydrolyze the amorphous fibril regions, thereby producing short rigid cellulose nanocrystals a few 100 nm in length. These nanocelluloses are of high technological interest due to their self-assembly into cholesteric liquid crystals, production of hydrogels or aerogels, use in nanocomposites with superior thermal and mechanical properties, and use as Pickering stabilizers for emulsions.
What is the most abundant organic polymer?
Cellulose is the most abundant organic polymer on Earth. The cellulose content of cotton fiber is 90%, that of wood is 40–50%, and that of dried hemp is approximately 57%. Cellulose is mainly used to produce paperboard and paper.
How many units of chain length is cellulose?
Cellulose from wood pulp has typical chain lengths between 300 and 1700 units; cotton and other plant fibers as well as bacterial cellulose have chain lengths ranging from 800 to 10,000 units. Molecules with very small chain length resulting from the breakdown of cellulose are known as cellodextrins; in contrast to long-chain cellulose, cellodextrins are typically soluble in water and organic solvents.
When was cellulose first used?
Cellulose was discovered in 1838 by the French chemist Anselme Payen, who isolated it from plant matter and determined its chemical formula. Cellulose was used to produce the first successful thermoplastic polymer, celluloid, by Hyatt Manufacturing Company in 1870. Production of rayon ("artificial silk ") from cellulose began in the 1890s and cellophane was invented in 1912. Hermann Staudinger determined the polymer structure of cellulose in 1920. The compound was first chemically synthesized (without the use of any biologically derived enzymes) in 1992, by Kobayashi and Shoda.
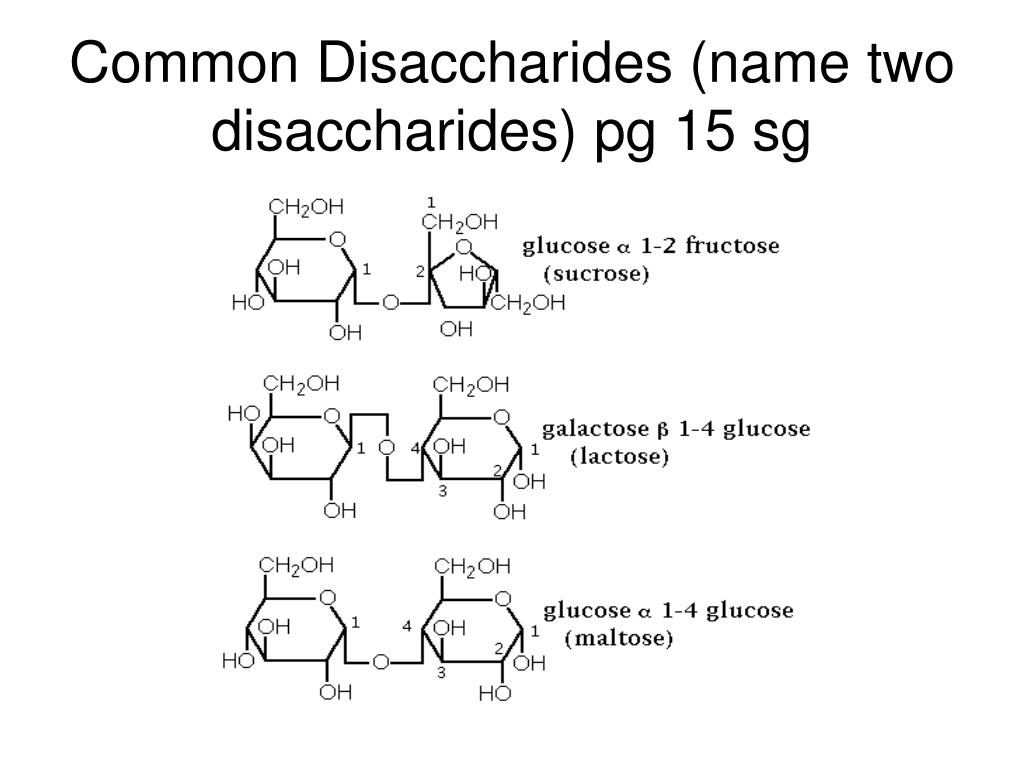
What bond connects glucose and galactose?
In lactose, the β-glycosidic bond connects galactose and glucose. What part of the structure identifies it as a β-glycosidic bond?
How do disaccharides form?
Disaccharides form when two monosaccharides undergo a dehydration reaction to form a glycosidic bond.
Why is sugar a reducing sugar?
It is a reducing sugar because it has free anomeric carbon.
Do potatoes have glycogen?
The storage polysaccharide glycogen is present in potatoes.
Overview
Processing
In plants cellulose is synthesized at the plasma membrane by rosette terminal complexes (RTCs). The RTCs are hexameric protein structures, approximately 25 nm in diameter, that contain the cellulose synthase enzymes that synthesise the individual cellulose chains. Each RTC floats in the cell's plasma membrane and "spins" a microfibril into the cell wall.
RTCs contain at least three different cellulose synthases, encoded by CesA (Ces is short for "cell…
History
Cellulose was discovered in 1838 by the French chemist Anselme Payen, who isolated it from plant matter and determined its chemical formula. Cellulose was used to produce the first successful thermoplastic polymer, celluloid, by Hyatt Manufacturing Company in 1870. Production of rayon ("artificial silk") from cellulose began in the 1890s and cellophane was invented in 1912. Hermann St…
Structure and properties
Cellulose has no taste, is odorless, is hydrophilic with the contact angle of 20–30 degrees, is insoluble in water and most organic solvents, is chiral and is biodegradable. It was shown to melt at 467 °C in pulse tests made by Dauenhauer et al. (2016). It can be broken down chemically into its glucose units by treating it with concentrated mineral acids at high temperature.
Hemicellulose
Hemicelluloses are polysaccharides related to cellulose that comprise about 20% of the biomass of land plants. In contrast to cellulose, hemicelluloses are derived from several sugars in addition to glucose, especially xylose but also including mannose, galactose, rhamnose, and arabinose. Hemicelluloses consist of shorter chains – between 500 and 3000 sugar units. Furthermore, hemicelluloses are branched, whereas cellulose is unbranched.
Regenerated cellulose
Cellulose is soluble in several kinds of media, several of which are the basis of commercial technologies. These dissolution processes are reversible and are used in the production of regenerated celluloses (such as viscose and cellophane) from dissolving pulp.
The most important solubilizing agent is carbon disulfide in the presence of alkali. Other agents include Schweizer's reagent, N-methylmorpholine N-oxide, and lithium chloride in dimethylacetamide. …
Commercial applications
Cellulose for industrial use is mainly obtained from wood pulp and from cotton.
• Paper products: Cellulose is the major constituent of paper, paperboard, and card stock. Electrical insulation paper: Cellulose is used in diverse forms as insulation in transformers, cables, and other electrical equipment.
• Fibers: Cellulose is the main ingredient of textiles. Cotton and synthetics (nylons) each …
See also
• Gluconic acid
• Isosaccharinic acid, a degradation product of cellulose
• Lignin
• Zeoform