What type of mixture is the salt and sand mixture?
heterogenous mixtureThe mixture of salt and sand is an example of heterogenous mixture as both components can be seen by naked eyes and easily separated.
What type of mixture is a mixture of sand and water?
SuspensionsSuspensions (heterogeneous) They are "suspended" in the liquid. A key characteristic of a suspension is that the solid particles will settle and separate over time if left alone. An example of a suspension is a mixture of water and sand. When mixed up, the sand will disperse throughout the water.
What kind of mixture is salt and water mixture?
homogeneous mixtureSaltwater acts as if it were a single substance even though it contains two substances—salt and water. Saltwater is a homogeneous mixture, or a solution.
Is mixture of sand and water a solution?
As sand is solid, it does not dissolve in water, forming 2 different phases present in the mixture of sand and water. Hence, it is a heterogeneous mixture.
Is sand water and salt a mixture?
Salt is soluble in water, which would give a homogeneous mixture. But, when we take sand, which is insoluble, we will have a mixture of two different phases, a solid and a liquid, therefore this is a heterogeneous mixture.
Is sand and water an example of homogeneous mixture?
The mixture of sand and water is heterogenous. We know that the mixture can be classified into two types namely, homogeneous mixture and heterogeneous mixture. We know that sand is solid and it does not dissolve in the water and it forms two different phases. Hence, the mixture of sand and water is heterogeneous.
What happens when you mix salt sand and water together?
Sand will remain undissolved at the bottom. Both salt and sand will dissolve completely in water.
Why salt and water is homogeneous mixture?
A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample.
How is sand and water is heterogeneous?
As sand is solid, it does not dissolve in water, forming 2 different phases present in the mixture of sand and water. Hence, it is a heterogeneous mixture.
What is sand and water solution called?
When sand is dissolved in water, it does not evenly mix with water and after some time it will settle down in the container. Therefore, the mixture of sand and water is heterogeneous mixture.
What type of mixture is sand and water solution suspension or colloid?
1: A mixture of sand and water forms a suspension. A suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon standing.
What is the mixture of sand and water and its uses?
Answer: Concrete is formed through a mixture of calcium oxide, water as well as sand and a few other gravel rocks. Concrete is a mixture because we can actually attain all the raw materials back from the hard concrete.
Is sand and water mixture colloid?
suspension/colloid. Sand in water is an example of . suspension/colloid.
Is sand and water mixture is suspension?
1: A mixture of sand and water forms a suspension. A suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon standing. The particles in a suspension are far larger than those of a solution, so gravity is able to pull them down out of the dispersion medium (water).
What is the mixture of sand and water and its uses?
Answer: Concrete is formed through a mixture of calcium oxide, water as well as sand and a few other gravel rocks. Concrete is a mixture because we can actually attain all the raw materials back from the hard concrete.
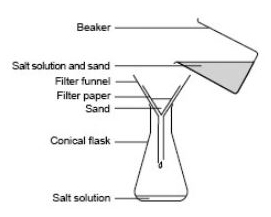
How to separate sand from salt water?
Another way you can separate the salt water and sand is to stir up the sand/salt water and pour it through a coffee filter to capture the sand.
How to make sand and salt?
Pour the salt and sand mixture into a pan. Add water. You don't need to add a lot of water. Solubility is a property that is affected by temperature, so more salt dissolves in hot water than cold water. It's okay if the salt doesn't dissolve at this point. Heat the water until the salt dissolves.
Why does salt not boil away with water?
The salt did not boil away with the water. This is because the boiling point of salt is much higher than that of water. The difference between boiling points can be used to purify water via distillation.
How to make sand out of salt water?
Remove the pan from heat and allow it to cool until it's safe to handle. Pour the salt water into a separate container. Now collect the sand.
What is the melting point of salt?
The melting point of salt is 1474°F (8 01°C), while that of sand is 3110°F (1710°C). Salt becomes molten at a lower temperature than sand. To separate the components, a mixture of salt and sand is heated above 801°C, yet below 1710°C. The molten salt may be poured off, leaving the sand. Usually, this is not the most practical method ...
What happens when you shake a pan of salt and sand?
If you shake a pan of salt and sand, the salt will eventually rise to the top. A similar method is used to pan for gold, since gold has a higher density than most other substances and sinks in a mixture .
Why do students separate salt and sand?
Students are often asked to separate salt and sand to learn about mixtures and to explore the differences between forms of matter that can be used to separate mixture components.