
Types of Distillation
- Simple Distillation. Simple distillation involves heating the liquid mixture to the boiling point and immediately...
- Fractional Distillation. Fractional distillation is often used to separate mixtures of liquids that have similar boiling...
- Steam Distillation. Steam distillation is often used to separate heat-sensitive components in a mixture. This...
What are the six ways of separating mixtures?
· Fractional distillation is a method for separating a liquid from a mixture of two or more liquids. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. This method works because the liquids in the mixture have different boiling points. Click to see full answer.
Which mixtures can be separated by distillation?
· Generally, distillation is used to separate mixtures that are homogeneous. What type of mixture is chromatography? Chromatography is a technique to separate components of a homogeneous mixture based on the solubility difference of the components in a solvent or solvent mixture. Typically it is performed in liquid or in gas phase.
What are three ways to separate a mixture?
· There are two basic processes for separating mixtures, distillation and filtration. In general, these are applied for the separation of homogeneous and heterogeneous mixtures, respectively. These can be separated by distillation in a laboratory using a burner placed under a beaker containing the salt water.
What techniques can be used to separate mixtures?
· What types of mixture can be separate by simple distillation? Homogeneous Mixtures can be separated with distillation and Heterogeneous can be separated by filtration.
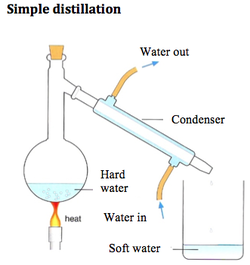
What type of mixtures are separated by simple distillation?
Simple distillation is a method for separating the solvent from a solution. For example, water can be separated from salt solution by simple distillation. This method works because water has a much lower boiling point than salt. When the solution is heated, the water evaporates.
What two types of mixtures can distillation separate?
Fractional distillation is a method for separating a liquid from a mixture of two or more liquids. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation.
What mixtures use distillation?
Distillation is used for many commercial processes, such as the production of gasoline, distilled water, xylene, alcohol, paraffin, kerosene, and many other liquids. Gas may be liquefied and separate. For example: nitrogen, oxygen, and argon are distilled from air.
Can distillation separate heterogeneous mixtures?
There are two basic processes for separating mixtures, distillation and filtration. In general, these are applied for the separation of homogeneous and heterogeneous mixtures, respectively.
What are the types of distillation?
The most commonly used techniques are simple distillation, fractional distillation, steam distillation, and vacuum distillation. In simple distillation process, a volatile compound is evaporated and channeled through a distillation column into a condenser, where it is eventually captured.
What is distillation and examples?
1. The simplest and most common example of distillation is the steam from a kettle, which gets deposited as drops of distilled water on a cold surface. 2. This process is used in the separation of alcoholic liquors from fermented materials for purification of alcohol by separating liquids from non-volatile solids.
Can sugar and water be separated distillation?
Let's consider a sugar solution which is a pure mixture of water and sugar. The sugar can be separated by removing the water from the solution. Therefore, distillation is the process by which sugar can be separated from the sugar solution.
Can oil and water be separated by distillation?
Distillation Methods The first uses a high-boiling-point water-immiscible liquid such as a mineral oil. The sample is suspended in this oil and heated to a temperature high enough to allow the water to be vaporized. As the water is distilled off, it is condensed and collected in a vessel and measured.
What are some examples of distillation?
Salt water is turned into fresh water through distillation. Various forms of fuel, such as gasoline, are separated from crude oil by distillation. Alcoholic beverages are made through distillation. The alcohol is boiled off from the rest of the mixture and collected in a concentrated format.
What is distillation for which type of mixture distillation is used?
Distillation is a widely used method for separating mixtures based on differences in the conditions required to change the phase of components of the mixture. To separate a mixture of liquids, the liquid can be heated to force components, which have different boiling points, into the gas phase.
What type of mixture can be separated by crystallization?
"Crystallization is also a chemical solid-liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs." Therefore, crystallization is used to separate a solid-liquid mixture.
Can all homogeneous mixtures be separated by distillation?
Homogenous mixtures also called solutions can be separated into the constituent substances by distillation if there is a difference in the concentration of the constituent substances in the gaseous phase. In cases where this does not happen, distillation cannot be used as a means of separation.
What mixtures can be separated by chromatography?
Separating dissolved solids – chromatography. Paper chromatography is a method for separating dissolved substances from one another. It is often used when the dissolved substances are coloured, such as inks, food colourings and plant dyes.
What are some mixtures that can be separated?
For example, air, sea water, crude oil, etc. The constituents of a mixture can be separated by physical means like filtration, evaporation, sublimation and magnetic separation.
What type of mixtures can be separated by solvent extraction?
Liquid–liquid extraction (LLE), also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (non-polar).
How are mixtures separated?
mixtures can be separated using various separation methods such filtration ,separating funnel,sublimation,simple distillation and paper chromatography.
What is fractional distillation?
Fractional distillation is a method for separating a liquid from a mixture of two or more liquids. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. This method works because the liquids in the mixture have different boiling points. Click to see full answer.
What is distillation used for?
Simple distillation is used to separate salt from seawater, to separate sugar from water and to separate ethanol from water in the production of hard liquor. One may also ask, can all homogeneous mixtures be separated by distillation?
What is distillation in chemistry?
What is Distillation? Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. It is a separation technique that can be used to either increase the concentration of a particular component in the mixture or to obtain (almost) pure components from the mixture.
What is fractional distillation?
Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. It involves several vaporization-condensation steps (which takes place in a fractioning column). This process is also known as rectification.
Is distillation a chemical reaction?
It is important to note that distillation is not a chemical reaction but it can be considered as a physical separation process.
Is distillation continuous or continuous?
The distillation performed on a laboratory scale often uses batches of the liquid mixture whereas industrial distillation processes are generally continuous, requiring a constant composition of the mixture to be maintained.
What is the partial pressure of a liquid?
As per Raoult’s law, the partial pressure of a single liquid component in an ideal liquid mixture equals the product of the vapor pressure of the pure component and its mole fraction. According to Dalton’s law of partial pressures, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures ...
What is the total pressure of a mixture of gases?
According to Dalton’s law of partial pressures, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of all the constituent gases. When a mixture of liquids is heated, the vapor pressure of the individual components increases, which in turn increases the total vapor pressure.
What happens at the boiling point of a mixture of liquids?
At the boiling point of a mixture of liquids, all the volatile constituents boil. However, the quantity of a constituent in the resulting vapor is based on its contribution to the total vapor pressure of the mixture. This is why the compounds with higher partial pressures can be concentrated in the vapors whereas the compounds having low partial ...
What is the process of distillation called?
Vacuum Distillation. The distillation process can be classified as atmospheric distillation and vacuum distillation. The distillation process which is conducted under vacuum or negative pressure is known as vacuum distillation.
What is distillation in science?
Distillation can be defined as the process of separating components or substances from the mixture using different boiling points or relative volatility. In other words, the distillation Process involving the conversion of a liquid into a vapor that is subsequently condensed back to liquid form and herby separates one liquid from another.
What is distillation in manufacturing?
Distillation is a process of liquid evaporation and converted into vapor and recondense and collected in a vessel. In the manufacturing process, distillation is a commonly used unit operation as a part of the solvent recovery process (SR Plant), impurity removal process or purification process, and many more.
What is the difference between atmospheric distillation and vacuum distillation?
It is similar to atmospheric distillation but the only difference is vacuum distillation is conducted under vacuum. The boiling point is directly proportional to pressure. If the boiling point of any substance present in the mixture is high, in that case we need to use utility which can help to achieve the boiling point of that high boiling component which will be costly. So to avoid using that utility, vacuum distillation is used in which vacuum is created in the column which will decrease the boiling point of component and hence separation can be achieved at low temperature.
How does fractional distillation work?
In fractional distillation, so the mixture is heated and the low boiling substance starts evaporating first, condenses the liquid first, and separate out. Now increase the temperature and similarly separate out the components from lower boiling point to higher boiling point.
What is azeotropic distillation?
Azeotropic Distillation. Azeotropic distillation is carried out when we have a mixture of immisible liquid which cannot be seperated using simple distillation as the diffence in boling point is very low and such mixtures are called as azeotropic mixture.
What is simple distillation?
Simple Distillation. Simple distillation is a procedure by which two liquids with different boiling points can be separated. Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at least fifty degrees difference in their boiling points. As the liquid being distilled is heated, ...
What is distillation process?
What are the different types of distillation process? Distillation is a physical method of assorting mixtures depending upon the difference in the boiling point of the component substances. In simple words, the working principle of distillation is to heat a mixture at a specific temperature, collect the hot vapors, ...
How to separate two liquids with different boiling points?
Simple distillation is a procedure by which two liquids with different boiling points can be separated. Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at least fifty degrees difference in their boiling points. As the liquid being distilled is heated, the vapors that form will be richest in the component of the mixture that boils at the lowest temperature. Purified compounds will boil, and thus turn into vapors, over a relatively small temperature range (2 or 3°C); by carefully watching the temperature in the distillation flask, it is possible to affect a reasonably good separation.
What is fractional distillation?
Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. It involves several vaporization-condensation steps (which takes place in a fractioning column). This process is also known as rectification.
How is steam distillation carried out?
Steam distillation is carried out by passing dry steam through the plant material whereby the steam volatile compounds are volatilized, condensed and collected in receivers. Steam distillation has been in use for essential oil extraction for many years.
How does distillation work?
This is done by passing steam through the mixture (which is slightly heated) to vaporize some of it.
What is the process of separating crude oil?
Vacuum Distillation. Vacuum distillation is the technique used to separate higher boiling fractions of crude oil. The underlying theory and the process are analogous to those used to separate the lighter fractions in the atmospheric distillation process. The difference between the two physical separation methods is that atmospheric distillation ...
What is the process of separating a mixture?
Chromatography is the separation of a mixture by passing it in solution or suspension or as a vapor (as in gas chromatography) through a medium in which the components move at different rates. Thin-layer chromatography is a special type of chromatography used for separating and identifying mixtures that are or can be colored, especially pigments.
How are mixtures separated?
Mixtures can be separated using a variety of techniques. Chromatography involves solvent separation on a solid medium. Distillation takes advantage of differences in boiling points. Evaporation removes a liquid from a solution to leave a solid material. Filtration separates solids of different sizes.
How does distillation work?
Distillation is a purification process where the components of a liquid mixture are vaporized and then condensed and isolated. In simple distillation, a mixture is heated and the most volatile component vaporizes at the lowest temperature. The vapor passes through a cooled tube (a condenser), where it condenses back into its liquid state. The condensate that is collected is called distillate.
What is distillation in science?
In simple distillation, a mixture is heated and the most volatile component vaporizes at the lowest temperature. The vapor passes through a cooled tube (a condenser), where it condenses back into its liquid state. The condensate that is collected is called distillate.
How to separate homogenous mixtures?
Evaporation is a technique used to separate out homogenous mixtures where there is one or more dissolved solids. This method drives off the liquid components from the solid components. The process typically involves heating the mixture until no more liquid remains, Prior to using this method, the mixture should only contain one liquid component, unless it is not important to isolate the liquid components. This is because all liquid components will evaporate over time. This method is suitable to separate a soluble solid from a liquid.
How is table salt obtained?
This method is suitable to separate a soluble solid from a liquid. In many parts of the world, table salt is obtained from the evaporation of sea water. The heat for the process comes from the sun.
How did they isolate gold from the soil?
One of the approaches taken to isolate the gold from the soil was called “panning.”. Dirt would be placed in the pan and covered with water. After thorough mixing, the pan is gently swirled to remove dissolved material while the heavier gold settles ...
