
What is kevlar made of?
Kevlar. Kevlar is a heat-resistant and strong synthetic fiber, related to other aramids such as Nomex and Technora. Developed by Stephanie Kwolek at DuPont in 1965, this high-strength material was first commercially used in the early 1970s as a replacement for steel in racing tires.
Is Kevlar polyaromatic or amide?
CLUE #3: Kevlar is a polyaromatic amide. That is, it contains aromatic and amide groups. Other polymers with a high breaking strength often contain one or both of these molecular groups. CLUE #4: The individual polymer strands of Kevlar are held together by hydrogen bonds that form between the polar amide groups on adjacent chains.
What is crystallinity of Kevlar?
Crystallinity is obtained by a manufacturing process known as spinning, which involves extruding the molten polymer solution through small holes. The crystallinity of the Kevlar polymer strands contributes significantly to Kevlar's unique strength and rigidity. CLUE #3: Kevlar is a polyaromatic amide. That is, it contains aromatic and amide groups.
What is the tensile strength of Kevlar?
When Kevlar is spun, the resulting fiber has a tensile strength of about 3,620 MPa, and a relative density of 1.44. The polymer owes its high strength to the many inter-chain bonds. These inter-molecular hydrogen bonds form between the carbonyl groups and N H centers.
What molecules make up Kevlar?
A single Kevlar polymer chain could have anywhere from five to a million segments bonded together. Each Kevlar segment or monomer is a chemical unit that contains 14 carbon atoms, 2 nitrogen atoms, 2 oxygen atoms and 10 hydrogen atoms.
Are Kevlar polymers?
CLUE #1: KEVLAR is a long chain-like molecule known as a polymer, which consists of repeating units called monomers.
What kind of bonding is Kevlar?
hydrogen bondingKevlar has a highly crystalline structure. Strong covalent bonding in the fiber direction and weak hydrogen bonding in the transverse direction result in highly anisotropic properties of Kevlar fiber.
Is Kevlar a polymer or composite?
Kevlar is the trade name (registered by DuPont Co.) of aramid (poly-para-phenylene terephthalamide) fibers. Kevlar fibers were originally developed as a replacement of steel in automotive tires.
Is aramid a polymer?
aramid, in full aromatic polyamide, any of a series of synthetic polymers (substances made of long chainlike multiple-unit molecules) in which repeating units containing large phenyl rings are linked together by amide groups. Amide groups (CO-NH) form strong bonds that are resistant to solvents and heat.
Is Kevlar a condensation polymer?
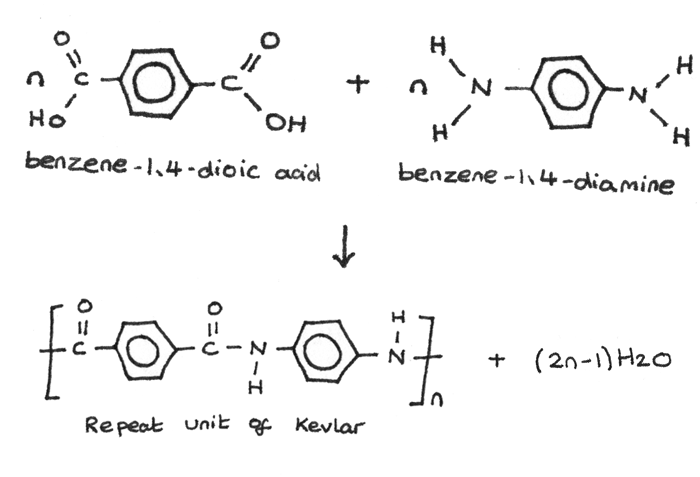
Kevlar is a nylon polymer and is obtained by condensation polymerization of terephthalic acid with 1,4-diamino benzene. It is a fibre used for making bullet proof vests.
What monomers is Kevlar made of?
Kevlar is produced by copolymerization of monomers benzene-1,4-diamine and terephthaloyl dichloride (an acid chloride derived from terephthalic acid). Polymerization occurs by the nucleophilic carbonyl substitution mechanism. Kevlar's strength is due in part to hydrogen bonding between the polymer chains.
What are the two categories of polymers?
Polymers fall into two categories: thermosetting plastic or thermoset. thermoforming plastic or thermoplastic.
What is the tensile strength of kevlar?
These chains are cross-linked with hydrogen bonds, providing a tensile strength 10X greater than steel on an equal weight basis.
Who makes Dupont kevlar?
Created by Stephanie Kwolek, DuPont ™ Kevlar ® is a heat-resistant para-aramid synthetic fiber with a molecular structure of many inter-chain bonds that make Kevlar ® incredibly strong.
What is the ballistic resistance of kevlar?
Ballistic resistance. Kevlar® fibers are so tightly spun that it is nearly impossible to separate them. When a bullet or other high-velocity projectile hits Kev lar®, the fibers essentially catch the projectile while absorbing and dissipating its energy.
Who is stronger than steel?
Stephanie Kwolek: stronger than steel. A pioneer in polymer research, Stephanie Kwolek accumulated several patents and awards throughout her career. Most notably, she is known for her groundbreaking work that led to the creation of Kevlar® - an incredibly strong material that continues to push the limits of possible.
What are the components of kevlar?
As you have discovered, there are many components that contribute to the strength of Kevlar, including the fact that Kevlar is a polymer containing aromatic and amide molecular groups. When the molten Kevlar is spun into fibers, the polymers have a crystalline arrangement, with the polymer chains oriented parallel to the fiber's axis. The amide groups are able to form hydrogen bonds between the polymer chains, which act like glue holding the separate polymer chains together. The most recent XANES images confirm that the aromatic components of Kevlar have a radial (spoke-like) orientation, which allows for a high degree of symmetry and order.
What is the name of the molecule that consists of repeating units called monomers?
CLUE #1: KEVLAR is a long chain-like molecule known as a polymer, which consists of repeating units called monomers.
Is kevlar a polyaromatic amide?
CLUE #3: Kevlar is a polyaromatic amide. That is, it contains aromatic and amide groups. Other polymers with a high breaking strength often contain one or both of these molecular groups.
What is the chemical composition of kevlar?
Kevlar’s chemical composition is nothing short of fascinating. Scientifically speaking, Kevlar is a poly-para-phenylene-terephthalamide (don’t worry, you aren’t the only one who can’t pronounce that). In human terms, it’s basically a type of plastic, but what makes Kevlar so strong is its molecular structure. Kevlar’s molecules are positioned parallel to each other and bound so tightly it makes the resulting material, quite literally, bulletproof. The tight molecule structure is what allows Kevlar to be so tear, abrasion, and heat-resistant, yet remain light and withstand extreme temperatures.
How is Kevlar Made?
Kevlar is made by producing the chemical compound first, then fusing it with another patented solution. Finally, the product is heated and spun, and the resulting fibers are then cut and woven into the desired fabric, be it ropes, bulletproof vests, or motorcycle jeans. The process of producing Kevlar is an extraordinary fusion of molecular chemistry and innovative design.
Who Invented Kevlar?
Polish-American scientist Stephanie Kwolek invented Kevlar in 1964. Kwolek was working for DuPont, a chemical company, at the time of Kevlar’s invention. While researching a solution for strong, heat-resistant car tires, Kwolek and her team made a discovery: blending a unique set of plastic polymers, Kwolek realized the resulting product was amazingly strong – much stronger than nylon which was widely used at the time. So much so, in fact, that DuPont immediately threw more resources at researching and developing this new material. By the late seventies, Kevlar was already in use in the car racing industry, then spread to aircraft and boat construction, and soon, protective clothing.
Where is Kevlar Used?
But it’s not just protective gear for humans: Kevlar is also widely used in manufacturing car and bicycle tires, aircraft, spacecraft, ropes, sails… the list is endless: whenever there’s a need for strength, heat resistance, cut and tear resistance, and durability, you’ll likely find Kevlar.
How Can Kevlar Improve Protection for Motorcycle Apparel?
Speaking of tough: while Kevlar is widely used as reinforcement for motorcycle jeans and ja ckets, often in the form of extra padding or extra layers, it can also reinforce other parts of the gear. One example is jeans and jacket seams. Burst resistance is something very few people ever think of, but consider this: even if your motorcycle jacket is made out of protective materials, it’s not going to be much help if it bursts at the seams when you crash. That’s where Kevlar comes in: here at Pando Moto. We love using Kevlar to reinforce the seams of our motorcycle jackets and pants to make sure the garment is virtually indestructible.
How fast can you drag a kevlar lining?
You can see an undamaged Kevlar lining during this 38 meters drag test at 45 km/h speed.
Is kevlar a high tensile material?
Because of its unique chemical composition and the process of synthesizing it, Kevlar is extremely high-tensile. It’s basically a fancy name for Kevlar’s signature property of being incredibly stretch- and tear-resistant.
What is the name of the monomer that is a 6 carbon chain with an amino group?
The other monomer is a 6 carbon chain with an amino group, -NH2, at each end. This is 1,6-diaminohexane (also known as hexane-1,6-diamine). When these two compounds polymerise, the amine and acid groups combine, each time with the loss of a molecule of water. This is known as condensation polymerisation.
How is nylon-6,6 made?
Nylon-6,6 is made by polymerising hexanedioic acid and 1,6-diaminohexane exactly as shown further up the page. Because the acid is acidic and the amine is basic, they first react together to form a salt. That is then converted into nylon-6,6 by heating it under pressure at 350°C.
What is the name of the acid that is converted into 1,6-diaminohexane?
That gives you the hexanedioic acid. Some of that can then be converted into the 1,6-diaminohexane. The acid is treated with ammonia to produce the ammonium salt. The ammonium salt is heated to 350°C in the presence of hydrogen and a nickel catalyst.
How many carbons are in nylon 6?
Nylon-6,6 is made from two monomers each of which contain 6 carbon atoms - hence its name. One of the monomers is a 6 carbon acid with a -COOH group at each end - hexanedioic acid. Note: When you count the carbons don't forget to include the ones in the -COOH groups. The other monomer is a 6 carbon chain with an amino group, -NH2, at each end. ...
What is the formula for polyamides?
Polyamides are polymers where the repeating units are held together by amide links. An amide grouphas the formula - CONH2. An amide linkhas this structure: In an amide itself, of course, the bond on the right is attached to a hydrogen atom.
What is a polyamide?
POLYAMIDES. This page looks at the structures, formation, hydrolysis and uses of the polyamides, nylon and Kevlar. What are polyamides? Polyamides are polymers where the repeating units are held together by amide links.
Is kevlar a monomer?
Kevlar is similar in structure to nylon-6,6 except that instead of the amide links joining chains of carbon atoms together, they join benzene rings. The two monomers are benzene-1,4-dicarboxylic acid and 1,4-diaminobenzene.
