What are the types of reactions undergone by alkynes?
- From alkyl halides ( Wurtz reaction)
- Decarboxylation of sodium salts of carboxylic compounds
- By hydrogenation of alkenes and alkynes
- By Frankland's reaction etc. Chemical properties of alkanes are
- Pyrolysis : formation of alkenes and alkynes
- Oxidation through: oxygen , potassium dichromate / conc. sulfuric acid , manganese dioxide , Copper tube et
Why are alkenes more reactive than the alkane?
Alkenes are relatively stable compounds, but are more reactive than alkanes because of the reactivity of the carbon–carbon π-bond. Most reactions of alkenes involve additions to this π bond, forming new single bonds. Click to read further detail. Correspondingly, which one is more reactive alkane or alkene?
Why do Alkenes undergo electrophilic addition reactions?
Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. To produce a more stable saturated product, alkenes undergo an addition reaction. Hybridization shifts from sp2 to sp3 in this reaction. A substitution process maintains the resonance stability of arene.
Is alkene is more reactive than alkyne?
alkenes is more reactive than alkynes and alkynes are more reactive than alkanes .the reason is alkenes contain double bond and pi electrons so addition reactions can take place. Alkynes have 3 double bond so it does go under addition reaction but alkanes contain a strong single sigma bond only goes under substitution reaction in presence of light.
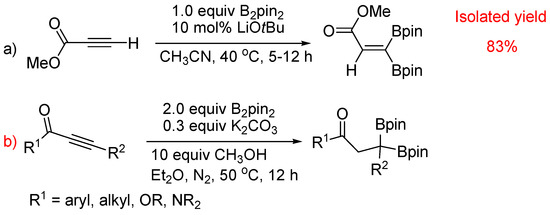
Do alkynes undergo substitution reactions?
Alkanes undergo only substitution reactions but alkenes and alkynes undergo both substitution and addition reactions.
What type of reaction do alkenes undergo?
Addition ReactionsAddition Reactions. Most reactions that occur with alkenes are addition reactions. As the name implies, during an addition reaction a compound is added to the molecule across the double bond. The result is loss of the double bond (or alkene structure), and the formation of the alkane structure.
Do alkynes undergo combustion reaction?
Alkynes, like other hydrocarbons, undergo combustion, producing carbon dioxide and water (with complete combustion) or mixtures of carbon dioxide, carbon monoxide, carbon, and water (with incomplete combustion). Alkynes undergo a number of addition reactions, as shown in the following table.
Do alkynes undergo electrophilic reactions?
Why do alkynes undergo electrophilic addition reactions ? The electrophilic addition reactions take place in alkynes due to the presence of high electron density in the triple bond. This means that – C ≡ C – can act as a source of electrons.
Which type of reaction occurs in alkanes?
General Reaction of Alkanes Alkane halogenation is an example of a substitution reaction, a type of reaction that often occurs in organic chemistry. A substitution reaction is a chemical reaction in which part of a small reacting molecule replaces an atom or a group of atoms on a hydrocarbon or hydrocarbon derivative.
What type of reaction is alkene to alkane?
hydrogenationIntroduction. An example of an alkene addition reaction is a process called hydrogenation.In a hydrogenation reaction, two hydrogen atoms are added across the double bond of an alkene, resulting in a saturated alkane.
What are the four types of reactions shown by alkynes?
There are many types of reactions that can happen with alkynes:Reduction - add molecules of hydrogen across double and triple bonds.Halogenation - adds a bromine, chlorine, or iodine atom.Alkylation - adds an alkyl group onto the molecule.Oxidation - adds oxygen to the molecule.
Which type of reaction takes in the multiple bonds of alkynes and alkene?
electrophilic addition reactionsAs a rule, electrophilic addition reactions to alkenes and alkynes proceed by initial formation of a pi-complex, in which the electrophile accepts electrons from and becomes weakly bonded to the multiple bond.
Does ethyne undergo addition reaction?
ethene is an unsaturated hydrocarbon ,it undergoes addition reaction to become saturated hydrocarbon whereas ethane is saturated hydrocarbon therefore it does not undergo addition reaction. triple bond which are generally weaker than C single bond. these reactive site in ethyne which can give easily addition reaction.
Is alkyne an electrophile or nucleophile?
"The clouds of electrons surrounding the sigma bond makes an alkyne an electron-rich molecule. They are therefore nucleophiles that react with electrophiles. Thus alkynes, like alkenes, undergo electrophilic addition reactions because of their weak pi bonds.
Do alkynes react as nucleophile?
Conjugate base anions of terminal alkynes (acetylide anions) are nucleophiles, and can do both nucleophilic substitution and nucleophilic addition reactions.
What is electrophilic addition reaction of alkynes?
What is electrophilic addition reaction with example? Electrophiles are the electron deficiency compound. In the alkyne there are one sigma bond and two pi bonds between two carbon atoms. These pi bonds are electron rich species; they form bonds with electrophile to form electrophilic addition reactions.
Do alkenes undergo elimination reactions?
Nucleophilic substitution (SN) reactions frequently compete with elimination reactions. The carbon skeletons of carbocations formed during E1 reactions sometimes rearrange. Elimination reactions form alkenes as well as alkynes. This section describes alkene-forming eliminations.
What type of reaction will an alkene not undergo?
The answer is e)Dehydration.
Why do alkenes undergo addition reactions?
Alkenes are unsaturated molecules, which means they do not have all the hydrogen they could have. This is because there is at least one double bond between carbons. This is a stable structure, but not the most stable, so when certain compounds or elements are added, like fluorine, they undergo an addition reaction.
Do alkenes undergo electrophilic substitution?
Thus, from the above discussion, it follows that alkenes undergo electrophilic addition reactions while arenes undergo electrophilic substitution reactions.
What are the four types of reactions shown by alkynes?
The four types of reaction include: 1. Reduction - add molecules of hydrogen across double and triple bonds. 2. Halogenation - adds a bromine, ch...
How do you find terminal alkynes?
A terminal alkyne is an alkyne in whose molecule there is at least one hydrogen atom bonded to a triply bonded carbon atom or simple the alkynes in...
What is the structure of alkynes?
Alkynes are hydrocarbons which contain carbon-carbon triple bonds. Their general formula is CnH2n-2 for molecules with one triple bond (and no rin...
Why are terminal alkynes important?
Terminal alkynes are unusual for simple hydrocarbons in that they can be deprotonated to generate a carbanion (i.e. a carbon atom bearing a negativ...
What are examples of terminal alkynes?
Terminal alkynes have the formula RC 2H. Examples include: diphenylacetylene and 3-hexyne; methylacetylene (propyne using IUPAC nomenclature; acety...
What are the major reactions that alkynes undergo?
You will then learn the major reactions that alkynes undergo: alkylation, reduction, addition, and oxidation.
What is the reaction of a terminal alkyne?
Alkylation reaction of a terminal alkyne. Sodium amide is used (1), then the deprotonated alkyne is reacted with a haloalkane (2).
How are haloalkanes formed?
Haloalkanes can be formed from alkynes in two types of reactions. In a halogenation reaction, where one or more hydrogen atoms are replaced by a halogen, molecular bromine is added to the alkene. This adds a bromine atom to each side of the triple bond, forming a haloalkene, which reacts again to form the compound shown, containing four bromine atoms.
What is the name of the reaction that adds an alkyl group to a molecule?
Alkylation is the addition of an alkyl group to a molecule, which can be an alkane. Terminal alkynes can undergo alkylation reactions because the H atom on them is somewhat acidic, and therefore willing to donate its proton. The H atom of a terminal alkyne is removed by a relatively strong base.
What are alkanes made of?
Alkanes are similar to alkynes, in that they are made up of carbon and hydrogen atoms , but alkanes contain only single bonds.
Where are alkynes located in a molecule?
Alkynes can be internal alkynes or terminal alkynes. Internal alkynes, just like they sound, are in the center of a molecule. Terminal alkynes are at the end, and contain an H atom on at least one of the triply bonded carbon atoms.
Can alkanes be formed from reduction reactions?
We can form both alkanes and alkenes from reduction reactions. Let take a look at three reactions.
Reactions of Alkenes and Alkynes
Alkenes and alkynes are generally more reactive than alkanes due to the electron density available in their pi bonds. In particular, these molecules can participate in a variety of addition reactions and can be used in polymer formation.
Addition Reactions
Unsaturated hydrocarbons can participate in a number of different addition reactions across their double or triple bonds.
Cycloaddition
Alkenes undergo diverse cycloaddition reactions. Most notable is the Diels–Alder reaction with 1,3-dienes to give cyclohexenes.
Oxidation
Oxidation of alkynes by strong oxidizing agents such as potassium permanganate or ozone will yield a pair of carboxylic acids. The general reaction can be pictured as:
Hydrogenation
In the presence of a catalyst—typically platinum, palladium, nickel, or rhodium—hydrogen can be added across a triple or a double bond to take an alkyne to an alkene or an alkene to an alkane.
Halogenation
Alkenes and alkynes can also be halogenated with the halogen adding across the double or triple bond, in a similar fashion to hydrogenation. The halogenation of an alkene results in a dihalogenated alkane product, while the halogenation of an alkyne can produce a tetrahalogenated alkane.
Hydrohalogenation
Alkenes and alkynes can react with hydrogen halides like HCl and HBr. Hydrohalogenation gives the corresponding vinyl halides or alkyl dihalides, depending on the number of HX equivalents added. The addition of water to alkynes is a related reaction, except the initial enol intermediate converts to the ketone or aldehyde.
Answer
alkenes undergo combustion [burning]in a similar way to alkanes. they also undergo many other chemical reaction that alkanes do -not double bond in alkenes makes them more reactive than alkanes
Answer
Alkanes undergo only substitution reactions but alkenes and alkynes undergo both substitution and addition reactions