The drawbacks of Newlands Law of Octaves are as following: Out of the total 56 known elements, Newland could arrange elements only up to calcium. Every eighth element did not show properties similar to that of the first after calcium. Just 56 elements were known at the time of Newlands, but afterwards, various elements were discovered.
What are the drawbacks of Newland's law of octaves?
The drawbacks of Newland's law of octaves are as follows: There was no proper place for hydrogen. Transition elements were not considered for octaves. The observation made by Newland does not hold well for those elements which lying beyond Ca. After Calcium,every eighth element did not possess properties similar to that of the first.
What are the limitations of the law of octaves?
Limitations of law of octaves: The law of octaves has the following limitations ; (A) The law of octaves was found to be applicable only upto calcium. It was not applicable to elements of higher atomic masses. ... (C) Newlands placed two elements in the same slot to fit elements in the table.
Why is it called the law of octaves?
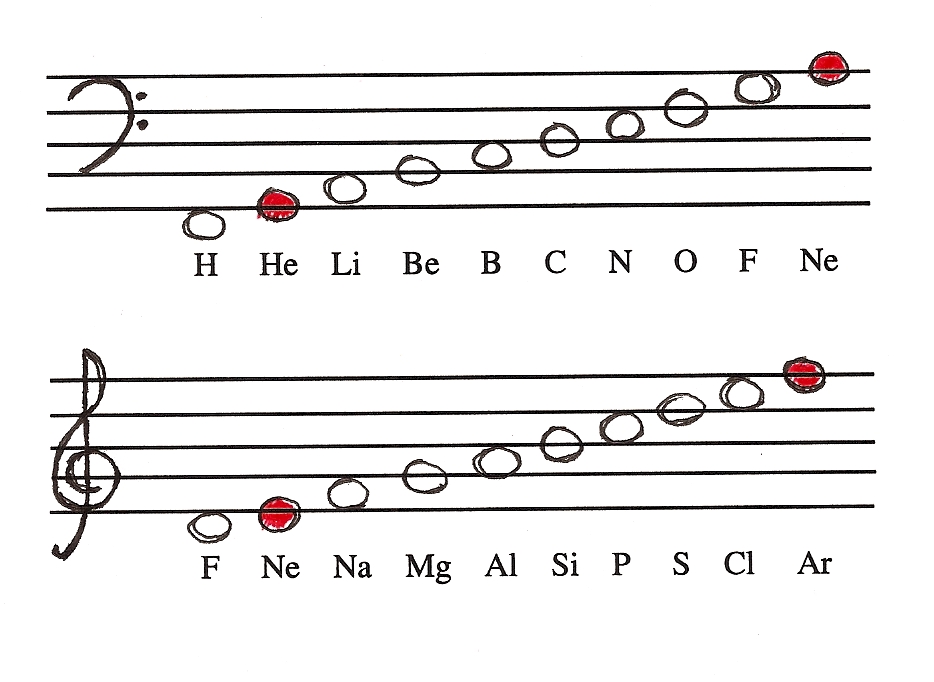
Newlands Law Was Named the Law of Octaves. In 1866, he observed that when elements were arranged to increase atomic masses, there was much similarity in the properties of every eighth element like the musical notes do, re, me, etc. Newland named this repetition as the law of octaves.
Why does the law of octaves fail to incorporate transition metals?
Hence, both elements share the same physical and chemical properties. The remaining elements after potassium (Cu, Rb, Ag, and a few more) are different. Those elements do not show similarities to the former elements. This is the reason why the law of octaves fails to incorporate transition metals.
Why did the law of octaves fail?
The law failed because of the following reasons: 1 The law was applicable only upto calcium. It could not include the other elements beyond calcium. ii With the discovery of rare gases it was the ninth element and not the eighth element having similar chemical properties.
What was the drawback of the law of octaves?
Q. What are the drawbacks of Newland's Law of Octaves? (A) The law of octaves was found to be applicable only till calcium. It was not applicable to elements of higher atomic masses.
What were the problems with Newlands octaves?
Newlands' table showed a repeating or periodic pattern of properties , but this pattern eventually broke down. By ordering strictly according to atomic mass, Newlands was forced to put some elements into groups which did not match their chemical properties.
What were the 3 limitations of Newlands law of octaves?
a) It was not applicable throughout the arrangements. It was applicable up to calcium only. The properties of the elements listed after calcium showed no resemblance to the properties of the elements above them. b) Those elements that were discovered after Newlands' octaves did not follow the law of octaves.
What was Newlands law of octaves and its drawbacks?
The drawbacks of Newland's law of octaves are as follows: Out of the total 56 known elements, Newland could arrange elements only up to calcium. Every eighth element did not show properties similar to that of the first after calcium.
What are the advantages and disadvantages of Newlands law of octaves?
Advantage-This law helped to arrange the elements with similar properties and provided a basis for classification. Disadvantage-This law is only applicable up to Calcium as only 56 elements existed at the time he made this law.
What was the law of octaves?
law of octaves, in chemistry, the generalization made by the English chemist J.A.R. Newlands in 1865 that, if the chemical elements are arranged according to increasing atomic weight, those with similar physical and chemical properties occur after each interval of seven elements.
What were the limitations of Newlands law of octaves Brainly?
The major limitations were: It applied to only lighter elements having atomic masses up to 40 u, i.e., up to calcium. The first and eighth elements after calcium did not have the same properties. Only 63 elements were considered to exist in nature, and no new elements would be discovered in the future.
Why were scientists critical of the law of octaves?
4. Why were many scientists critical about Newland's octaves? There was no clear division between metals and non-metals, there were no gaps left, he put two elements in one box, and elements in the same group had dissimilar properties. 5.
What were the drawbacks of Mendeleev periodic table?
The position of hydrogen was not defined correctly as it resembled few characteristics of alkali metals and few of halogens. Position of isotopes would be different according to Mendeleev's periodic table. No separate positions were given to lanthanoids and actinoids. Position of group VIII elements was not justified.
What are the drawbacks of modern periodic table?
Limitations of the modern periodic table:Uncertainty regarding the position of hydrogen whether it should be placed in the IA group or VIIA group. ... No place for isotopes of elements in the Modern periodic table.Lanthanides and Actinides are kept separately under the table not kept within the Modern periodic table.More items...
What are the drawbacks of dobereiner's law of triads?
The main Limitation of Dobereiner's classification of elements was that he failed to arrange all the then-known elements in the form of triads of elements having similar chemical properties. Dobereiner could identify only three triads. He was not able to prepare triads of all the known elements.
What was the law of octaves?
law of octaves, in chemistry, the generalization made by the English chemist J.A.R. Newlands in 1865 that, if the chemical elements are arranged according to increasing atomic weight, those with similar physical and chemical properties occur after each interval of seven elements.
What are the limitations of the law of octaves?
Limitations of law of octaves: The law of octaves has the following limitations ; (A) The law of octaves was found to be applicable only upto calcium. It was not applicable to elements of higher atomic masses. ... (C) Newlands placed two elements in the same slot to fit elements in the table.
Who discovered the law of octaves?
Law of octaves, in chemistry, the generalization made by the English chemist J.A.R. Newlands in 1865 that, if the chemical elements are arranged according to increasing atomic weight, those with similar physical and chemical properties occur after each interval of seven elements. Newlands was one of the first to detect a periodic pattern in the properties of the elements and anticipated later developments of the periodic law.
What was Newland's law of octaves?
Newland's law of octaves was among the very first attempts to detect a periodic pattern in the properties of elements.
Which law of octaves was also among the early attempts to classify elements on the basis of their?
Newland's law of octaves was also among the early attempts to classify elements on the basis of their atomic weights.
Which element followed the Newland law of octaves?
Newland’s law of octaves was applicable only to calcium. After that no element followed the law of octaves.
Can elements that were discovered later be fitted into the law of octaves?
The elements that were discovered later could not be fitted into the law of octaves.
Does Newland's law of octaves apply to elements heavier than calcium?
If you are talking about Newland's law of octaves, the biggest one is that it doesn't apply to elements heavier than Calcium.
How many shocks are there in an octave?
All processes are octaves and there are two shocks in the octave, one between the notes Mi and Fa and another between the Si of the preceding and the Do of the next octave. A special influx of energy or shock is needed for the octave to continue its progress over these special intervals.
What are shocks in music?
An octave has two special intervals called shocks. These are the ones between mi and fa and si and the do of the next octave. These correspond to the absence of the black key on the piano keyboard. A process proceeds through the stages denoted by the notes if it has enough initial impetus. However, the process does not keep its original direction if left to itself. Usually, the process also needs an extra impulse from outside to proceed past the shock between mi-fa or si-do. Lack of understanding of the Law of Seven is, according to the 4th Way, the principal reason why human plans almost never reach their goal and why activities usually turn into their antithesis. One example is revolution against tyranny automatically turning into more tyranny. The points of slowing down in the rate of increase of the rate of vibrations are called intervals.
What is the law of octaves?
State Law of Octaves. According to the law of octaves, each eighth element in the periodic table shares likewise physical and chemical properties. Thus, each element in the parallel row must have similar physical and chemical properties. But this is not true.
Who proposed the law of octaves?
Law of Octaves Was Proposed By. In chemistry, the law of octaves was proposed by the English chemist J.A.R. Newlands in 1865. Newlands was one of the first to notice a periodic pattern in the elements’ properties and predicted later developments of the periodic law.
What is Mendeleev's periodic law?
Mendeleev’s Periodic Law. The physical and chemical properties of elements of the periodic table are a periodic operation of their atomic masses. The periodic table is a table of known elements prepared so that elements with close properties occur in the same vertical column.
How did Newlands adjust the order of elements?
To adjust the existing element ordering, Newlands placed two elements in the same position, which differed in their chemical and physical properties.
Which law provided a broad scope to order all known elements into a tabular form?
The law provided a broad scope to order all known elements into a tabular form. Newlands law of octave was the first to logically based on the atomic weight, i.e., it links the elements’ properties to their atomic masses. This system worked quite better for the lighter elements.
Who discovered the triads of elements?
Ans. Dobereiner’s triads were groups of elements with similar properties identified by the German chemist Johann Wolfgang Dobereiner. Dobereiner noticed that a group of three elements (known as triads) could be identified in which all the elements shared having alike physical and chemical properties.
How many elements could Newland arrange?
Out of the total 56 known elements, Newland could arrange elements only up to calcium.
