
...
| Volume | Pressure | P x V |
|---|---|---|
| l2 | 117 9/16 | 1411 |
What is Boyle’s law science fair experiment?
Boyle’s Law Science Fair Experiment: Pressure vs. Volume of a Gas at Constant Temperature shows you a simple method for re-creating Boyle’s famous experiment. This is a modern version of a classic experiment by Robert Boyle on the compressibility of gases.
What was the purpose of the Boyle's Law Lab Report?
Boyles Law Lab Report 1. This experiment was meant to explore the relationship between pressure and gasses described by Boyles Law. This is important because the more pressure an object has, the less volume it has. Boyle's Law is identified through the equation PV=k.
What is Boyle's Law give an example?
Boyle's law demonstrations. The law itself can be stated as follows: For a fixed mass of an ideal gas kept at a fixed temperature, pressure and volume are inversely proportional. Or Boyle's law is a gas law, stating that the pressure and volume of a gas have an inverse relationship.
Who discovered Boyle's Law?
The law was named after chemist and physicist Robert Boyle, who published the original law in 1662. This relationship between pressure and volume was first noted by Richard Towneley and Henry Power in the 17th century. Robert Boyle confirmed their discovery through experiments and published the results.
/boylesdatagraphed-56a129b33df78cf77267fe5d.jpg)
What did Boyle's experiment lead to?
Known for his law of gases, Boyle was a 17th-century pioneer of modern chemistry. Every general-chemistry student learns of Robert Boyle (1627–1691) as the person who discovered that the volume of a gas decreases with increasing pressure and vice versa—the famous Boyle's law.
How did Boyle discover Boyle's law?
Boyle discovered the relationship between pressure and volume in a gas that is now known as Boyle's law by using a vacuum chamber to change the pressure in a gas. He made careful observations and conducted a series of experiments to show that pressure and volume are inversely proportional to each other.
What did Boyle's experiment teach him about gases?
Towneley's hypothesis.” Boyle's experiments confirmed what Towneley and Power had found: The pressure and volume of a gas, when held at a constant temperature, are inversely proportional. This means that when the volume of a container decreases, the pressure of the gas inside that container will increase.
What is the conclusion from Robert Boyle's experiment?
Conclusion: Robert Boyle, through experimentation, found the inverse relationship between gas pressure and gas volume for a given amount of gas at a constant temperature and atmospheric pressure.
Why is Boyle's law important?
Why is Boyle law important? Boyle's law is significant because it explains how gases behave. It proves beyond a shadow of a doubt that gas pressure and volume are inversely proportional. When you apply pressure on a gas, the volume shrinks and the pressure rises.
How do you do the Boyle's law experiment?
You can observe a real-life application of Boyle's Law when you fill your bike tires with air. When you pump air into a tire, the gas molecules inside the tire get compressed and packed closer together. This increases the pressure of the gas, and it starts to push against the walls of the tire.
When was Boyle's law discovered?
1662This empirical relation, formulated by the physicist Robert Boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; i.e., in equation form, pv = k, a constant. The relationship was also discovered by the French physicist Edme Mariotte (1676).
When did Boyle discover Boyle's law?
One of their findings, published in 1662, later became known as “Boyle's law.” This law expresses the inverse relationship that exists between the pressure and volume of a gas, and it was determined by measuring the volume occupied by a constant quantity of air when compressed by differing weights of mercury.
When did Boyle discover Boyle's law?
One of their findings, published in 1662, later became known as “Boyle's law.” This law expresses the inverse relationship that exists between the pressure and volume of a gas, and it was determined by measuring the volume occupied by a constant quantity of air when compressed by differing weights of mercury.
When was Boyle's law discovered?
1662This empirical relation, formulated by the physicist Robert Boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; i.e., in equation form, pv = k, a constant. The relationship was also discovered by the French physicist Edme Mariotte (1676).
When did Robert Boyle make his discovery?
In 1675 Boyle discovered that electric attraction operates in a vacuum.
Who was Robert Boyle influenced by?
Isaac NewtonRobert HookeDaniel BernoulliRichard LowerRobert Boyle/Influenced
How did Boyle study air?
Boyle systematically studied the compression of air, sealed in a glass tube with a U-shaped curve. The air was trapped by a column of mercury, added to the open end of the tube. By changing the amount of mercury in the tube, Boyle could change the pressure exerted on the trapped air.
Who discovered the relationship between pressure and volume of gases?
This project re-creates a study begun in 1662 by Robert Boyle.
What is the purpose of the first block in a syringe?
The first block will hold the syringe upright, and will need a hole that is just slightly larger than the diameter of the syringe, plus a smaller hole to accommodate the syringe tip. For greater stability, this block can be clamped in place (optional).
How to use a sealed syringe?
When your sealed syringe is ready for use, insert the syringe firmly, tip down, into the pre-drilled hole in the bottom wood block support, as shown in the diagram. The syringe should fit snugly, so it does not wobble when you load it up with bricks. You may wish to clamp the block in place. (Note: clamp to a workbench, not a piece of fine furniture!)
WHAT TO DO:-
First, trap a small amount of air in the balloon and tie a knot. Place the balloon in the syringe.
WHAT HAPPENS:-
When we place the bulb inside the syringe without squeezing the piston, the balloon remains the same as the air escapes from the front, keeping the atmospheric pressure the same. The moment we close the outlet of the syringe and squeeze the piston, the balloon becomes smaller under increased pressure.
WHY THIS HAPPENS:-
The balloon remains the same size when the pressure decreases and the volume increases. But when pressure increases the volume decreases therefore making the balloon compress to a smaller size.
What did Boyle believe about chemical experiments?
Boyle believed that chemical experiments could demonstrate the truth of the corpuscularian philosophy. In this context he defined elements in Sceptical Chymist (1661) as “certain primitive and simple, or perfectly unmingled bodies; which not being made of any other bodies, or of one another, are the ingredients of which all those called perfectly mixt bodies are immediately compounded, and into which they are ultimately resolved.”
What did Boyle's theory of material change do?
Boyle’s theories of material change did nothing to eliminate the possibility of the transmutation of base metals to gold that was at the heart of alchemy . Indeed he practiced alchemy until the end of his life, believed that he had witnessed transmutation, and successfully lobbied Parliament to repeal England’s ban on transmutation.
What is the second edition of Boyle's law?
The second edition of this work, published in 1662, delineated the quantitative relationship that Boyle derived from experimental values, later known as Boyle’s law: that the volume of a gas varies inversely with pressure.
Who reengraved the image of the air pump?
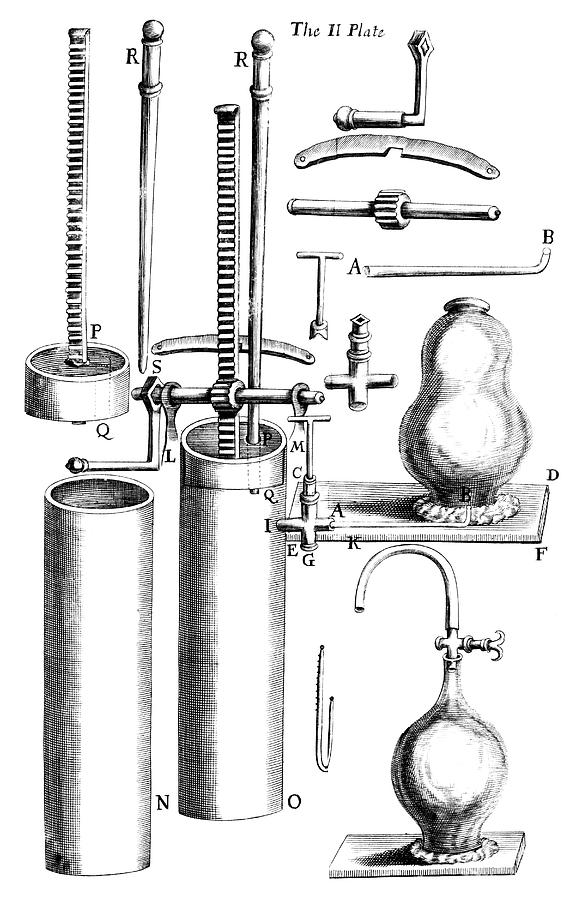
Boyle at the age of 37, with his air pump in the background. François Diodati reengraved this image from an engraving by William Fairthorne, Opera varia (1680).
Who was the first person to discover that the volume of a gas decreases with increasing pressure?
Robert Boyle. Every general-chemistry student learns of Robert Boyle (1627–1691) as the person who discovered that the volume of a gas decreases with increasing pressure and vice versa—the famous Boyle’s law. A leading scientist and intellectual of his day, he was a great proponent of the experimental method.
What is the name of the atomist who argued for corpuscularism?
Boyle was an advocate of corpuscularism, a form of atomism that was slowly displacing Aristotelian and Paracelsian views of the world. Instead of defining physical reality and analyzing change in terms of Aristotelian substance and form and the classical four elements of earth, air, fire, and water—or the three Paracelsian elements of salt, sulfur, ...
Experiment 2 – Boyles Law
The behaviour of a gas in an enclosed vessel can be described by four variables of pressure P , volume V , temperature T and the number of molecules n contained in the vessel. These variables are related to each other as shown in the ideal gas law equation below...
Boyles Law
In our experiment, our results firmly supported Boyle’s law. We found that a decrease in volume will increase pressure. In addition, multiplying temperature by pressure gave us values that were all very similar to each other. As a result, our results show us that the change in pressure and volume, while an inverse relationship, is nearly linear.
Who developed Boyle's law?
Daniel Bernoulli (in 1737–1738) derived Boyle's law by applying Newton's laws of motion at the molecular level. It remained ignored until around 1845, when John Waterston published a paper building the main precepts of kinetic theory; this was rejected by the Royal Society of England. Later works of James Prescott Joule, Rudolf Clausius and in particular Ludwig Boltzmann firmly established the kinetic theory of gases and brought attention to both the theories of Bernoulli and Waterston.
Who discovered the same law independently of Boyle?
The French physicist Edme Mariotte (1620–1684) discovered the same law independently of Boyle in 1679, but Boyle had already published it in 1662. Mariotte did, however, discover that air volume changes with temperature. Thus this law is sometimes referred to as Mariotte's law or the Boyle–Mariotte law.
What is the relationship between kinetic theory and ideal gases?
Boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its pressure. Most gases behave like ideal gases at moderate pressures and temperatures.
How was the law of motion derived?
Boyle (and Mariotte) derived the law solely by experiment. The law can also be derived theoretically based on the presumed existence of atoms and molecules and assumptions about motion and perfectly elastic collisions (see kinetic theory of gases ). These assumptions were met with enormous resistance in the positivist scientific community at the time, however, as they were seen as purely theoretical constructs for which there was not the slightest observational evidence.
What is the name of the law that describes how the pressure of a gas tends to decrease as the volume of?
Boyle's law, also referred to as the Boyle–Mariotte law, or Mariotte's law (especially in France), is an experimental gas law that describes how the pressure of a gas tends to decrease as the volume of the container increases. A modern statement of Boyle's law is:
Who discovered the relationship between pressure and volume?
This relationship between pressure and volume was first noted by Richard Towneley and Henry Power in the 17th century. Robert Boyle confirmed their discovery through experiments and published the results. According to Robert Gunther and other authorities, it was Boyle's assistant, Robert Hooke, who built the experimental apparatus. Boyle's law is based on experiments with air, which he considered to be a fluid of particles at rest in between small invisible springs. At that time, air was still seen as one of the four elements, but Boyle disagreed. Boyle's interest was probably to understand air as an essential element of life; for example, he published works on the growth of plants without air. Boyle used a closed J-shaped tube and after pouring mercury from one side he forced the air on the other side to contract under the pressure of mercury. After repeating the experiment several times and using different amounts of mercury he found that under controlled conditions, the pressure of a gas is inversely proportional to the volume occupied by it. The French physicist Edme Mariotte (1620–1684) discovered the same law independently of Boyle in 1679, but Boyle had already published it in 1662. Mariotte did, however, discover that air volume changes with temperature. Thus this law is sometimes referred to as Mariotte's law or the Boyle–Mariotte law. Later, in 1687 in the Philosophiæ Naturalis Principia Mathematica, Newton showed mathematically that in an elastic fluid consisting of particles at rest, between which are repulsive forces inversely proportional to their distance, the density would be directly proportional to the pressure, but this mathematical treatise is not the physical explanation for the observed relationship. Instead of a static theory, a kinetic theory is needed, which was provided two centuries later by Maxwell and Boltzmann .
Which law states that at constant temperature the volume of a given mass of a dry gas is inversely proportion?
Boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its pressure.
Who invented Boyle's law?
Boyle’s law was put forward by the Anglo-Irish chemist Robert Boyle in the year 1662. For a gas, the relationship between volume and pressure (at constant mass and temperature) can be expressed mathematically as follows. Where P is the pressure exerted by the gas and V is the volume occupied by it.
What is the result of Boyle's law?
This is accompanied by an increase in the pressure exerted by the air on the balloon, as a consequence of Boyle’s law. As the balloon is squeezed further, the increasing pressure eventually pops it.
What is the product of the initial pressure and the initial volume of a gas?
In other words, the product of the initial pressure and the initial volume of a gas is equal to the product of its final pressure and final volume (at constant temperature and number of moles). ...
Why is Boyle's law important?
Boyle’s law is significant because it explains how gases behave. It proves beyond a shadow of a doubt that gas pressure and volume are inversely proportional. When you apply pressure on a gas, the volume shrinks and the pressure rises.
What is the law of balloons?
A balloon is a good example of Boyle’s law in action. The balloon is inflated by blowing air into it; the pressure of the air pulls on the rubber, causing the balloon to expand. When one end of the balloon is compressed, the pressure within rises, causing the un-squeezed section of the balloon to expand outward.
Objective
Nomenclature
- Viis the volume reading of the gas in the air column.
- Piis the pressure reading in the pressure gauge.
Procedure
- Connect the apparatus as shown in the above diagram.
- The connection of the oil reservoir to the air column must be such that there is no leakage and the air is completely sealed off by the oil.
- The hand air pump is attached to the oil reservoir.
- After assembling all the types of equipment, open the air tap and start pumping air through t…
- Connect the apparatus as shown in the above diagram.
- The connection of the oil reservoir to the air column must be such that there is no leakage and the air is completely sealed off by the oil.
- The hand air pump is attached to the oil reservoir.
- After assembling all the types of equipment, open the air tap and start pumping air through the air pump.
Precaution
- The air in the column should be completely sealed off by the oil.
- The air pump is tightly connected to the oil reservoir.
- Each reading must be taken after two to three minutes of closing the air tap.
- The volume readings must be taken by levelling the eyes to the meniscus of the oil.
Result
- The graph of pressure vs volume and pressure vs inverse volume are plotted. As expected, the above graph (pressure vs volume) is an exponential curve. As observed from the graph, pressure decreases with rise in volume or vice versa. The graph of pressure to inverse volume is a straight line and follows the equation y = mx.
Conclusion
- The experiment is successfully studied, and the system obeys Boyle's law. From the graphs, it can be concluded that the pressure of the gas in the column is inversely proportional to the volume of the gas at a constant temperature for a fixed amount of gas.