Why did JJ Thomson discovered the electron?
Thomson, a highly-respected professor at Cambridge, determined the existence of electrons by studying cathode rays. He concluded that the particles making up the rays were 1,000 times lighter than the lightest atom, proving that something smaller than atoms existed.
What are facts about JJ Thompson?
Thomson
- Prior to Thomson's discovery of electrons, scientists believed the atom was the smallest fundamental unit of matter.
- Thomson called the particle he discovered 'corpuscles' rather than electrons.
- Thomson's master's work, Treatise on the motion of vortex rings, provides a mathematical description of William Thomson's vortex theory of atoms. ...
What is the conclusion of JJ Thomson experiment?
Conclusion. After completing the experiment J.J. Thomson concluded that rays were and are basically negatively charged particles present or moving around in a set of a positive charge. This theory further helped physicists in understanding the structure of an atom. And the significant observation that he made was that the characteristics of ...
What contributions did J.J. Thomson make to the atom?
Thomson
- #1 He did groundbreaking work in conduction of electricity in gases. ...
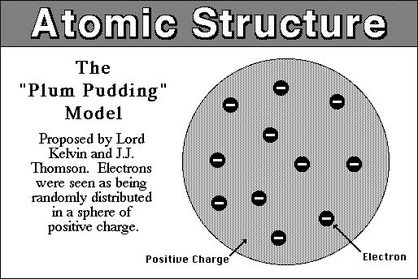
- #7 Thomson proposed the Plum Pudding Model of the atom. ...
- #8 His lectures foreshadowed Einstein's Quantum Theory of Light. ...
- #9 J J Thomson made vital contributions to Mass Spectrometry. ...
See more

When did Thomson find the electron?
1897During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Their work culminated in the discovery by English physicist J.J. Thomson of the electron in 1897.
How did Thomson discover the electron?
Thomson discovered the electron by experimenting with a Crookes, or cathode ray, tube. He demonstrated that cathode rays were negatively charged. In addition, he also studied positively charged particles in neon gas.
What did J.J. Thomson discover in 1906?
In 1906, Thomson demonstrated that hydrogen had only a single electron per atom. Previous theories allowed various numbers of electrons.
What 3 things did J.J. Thomson discover?
J. J. Thomson took science to new heights with his 1897 discovery of the electron – the first subatomic particle. He also found the first evidence that stable elements can exist as isotopes and invented one of the most powerful tools in analytical chemistry – the mass spectrometer.
What 2 Things did JJ Thomson discover?
In 1897 Thomson discovered the electron and then went on to propose a model for the structure of the atom. His work also led to the invention of the mass spectrograph.
Who invented the electron?
J.J. ThomsonJ.J. Thomson decided to find out for sure. Thomson was a physics professor at Cambridge University in the UK. He placed cathode tubes in electric and magnetic fields.
Who discovered electrons in 1897?
physicist J.J. ThomsonOn April 30, 1897, British physicist J.J. Thomson announced his discovery that atoms were made up of smaller components. This finding revolutionized the way scientists thought about the atom and had major ramifications for the field of physics.
What did JJ Thomson call electrons?
corpusclesThomson determined that all matter is made up of tiny particles that are much smaller than atoms. He originally called these particles 'corpuscles,' although they are now called electrons.
Who discovered the atom?
chemist John DaltonIn 1808, chemist John Dalton developed a very persuasive argument that led to an amazing realization: Perhaps all matter (i.e., stuff, things, objects) is made of tiny, little bits. Fundamental bits. Indivisible bits. Atomic bits.
Who is the father of electron?
Sir Joseph John ThomsonThomson, in full Sir Joseph John Thomson, (born December 18, 1856, Cheetham Hill, near Manchester, England—died August 30, 1940, Cambridge, Cambridgeshire), English physicist who helped revolutionize the knowledge of atomic structure by his discovery of the electron (1897).
Who was the father of proton?
Ernest RutherfordThe proton was discovered by Ernest Rutherford in the early 1900's. During this period, his research resulted in a nuclear reaction which led to the first 'splitting' of the atom, where he discovered protons. He named his discovery “protons” based on the Greek word “protos” which means first.
What is J.J. Thomson theory?
Summary. J.J. Thomson's experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. Thomson proposed the plum pudding model of the atom, which had negatively-charged electrons embedded within a positively-charged "soup."
How did J.J. Thomson find the mass of an electron?
Thomson was able to deflect the cathode ray towards a positively charged plate deduce that the particles in the beam were negatively charged. Then Thomson measured how much various strengths of magnetic fields bent the particles. Using this information Thomson determined the mass to charge ratio of an electron.
How did J.J. Thomson's experiment work?
J.J. Thomson's first experiment with cathode rays involved an electrometer and a magnet. By bending the cathode rays away from the electrometer he was able to determine that cathode rays and the electric charges were one and the same.
What did J.J. Thomson discover and how?
On April 30, 1897, British physicist J.J. Thomson announced his discovery that atoms were made up of smaller components. This finding revolutionized the way scientists thought about the atom and had major ramifications for the field of physics.
Why did J.J. Thomson do his experiment?
He decided to try to work out the nature of the particles. They were too small to have their mass or charge calculated directly, but he attempted to deduce this from how much the particles were bent by electrical currents, of varying strengths.
What did Thomson discover about neon?
Next to this famous discovery, Thomson and his research assistant F. W. Aston channeled a stream of neon ions through a magnetic and an electric field. They measured its deflection and observed two patches of light on the photographic plate, which was placed in the path. They concluded, that neon is composed of atoms of two different atomic masses ( isotopes ), which was the first evidence for isotopes of a stable element. Also, Thomson’s separation of neon isotopes by their mass was the first example of mass spectrometry. In 1905, Thomson discovered the natural radioactivity of potassium and one year later he managed to demonstrate that hydrogen had only a single electron per atom.
What did Thomson believe about the structure of the electron?
Thomson believed that the corpuscles emerged from the atoms of the trace gas inside his cathode ray tubes. He concluded that atoms were divisible, and that the corpuscles were their building blocks.
What did Thomson find about the cathode rays?
As a result, Thomson could suggest that cathode rays were more than 100 times lighter than the hydrogen atom and also, he concluded that their mass was the same in whichever type of atom they came from. He then concluded that the rays were composed of light and negatively charged particles, a universal building block of atoms. Thomson named the particles “corpuscles”, but scientists preferred the name electron which had been suggested by George Johnstone Stoney prior to Thomson’s discovery. One month after Thomson’s important announcement of the corpuscle he found that he could reliably deflect the rays by an electric field if he evacuated the discharge tube to a very low pressure. By comparing the deflection of a beam of cathode rays by electric and magnetic fields he obtained more robust measurements of the mass to charge ratio that confirmed his previous estimates. This became the classic means of measuring the charge and mass of the electron. Thomson believed that the corpuscles emerged from the atoms of the trace gas inside his cathode ray tubes. He concluded that atoms were divisible, and that the corpuscles were their building blocks. To explain the overall neutral charge of the atom, he proposed that the corpuscles were distributed in a uniform sea of positive charge. His model became widely known as the “ plum pudding” model of atoms. Ernest Rutherford disproved this model later on with his famous gold foil experiment, which led to the discovery of the nucleus . [ 4 ]
What was the first example of mass spectrometry?
Also, Thomson’s separation of neon isotopes by their mass was the first example of mass spectrometry. In 1905, Thomson discovered the natural radioactivity of potassium and one year later he managed to demonstrate that hydrogen had only a single electron per atom.
What are rays made of?
He then concluded that the rays were composed of light and negatively charged particles, a universal building block of atoms. Thomson named the particles “corpuscles”, but scientists preferred the name electron which had been suggested by George Johnstone Stoney prior to Thomson’s discovery.
What are the two isotopes of neon?
In the bottom right corner of this photographic plate are markings for the two isotopes of neon: neon-20 and neon-22.
What was the Nobel Prize for Physics in 1906?
In 1906 he was awarded the Nobel Prize in Physics for his research on the electrical conductivity of gases. Thomson was awarded a Knight Bachelor’s degree in 1908 and was admitted to the Order of Merit in 1912. Since 1907 he was a corresponding member of the Bavarian Academy of Sciences.
What is the atom composed of?
According to this model, an atom consist of a sphere of positive matter within which electrostatic forces determined the positioning of the negatively charged corpuscles. This fact explains the overall neutral charge of the atom, Thomson proposed that the corpuscles were distributed in a uniform sea of positive charge, in which they are scattered like “the plums in a pudding”.
What was the smallest division of matter?
For almost 100 years (from Dalton’s A New System of Chemical Philosophy) atoms were thought to be the smallest possible division of matter. All of the results of chemical experiments during this time indicated that the atom was indivisible. Eventually, experimentation into electricity and radioactivity indicated that particles of matter smaller than the atom did indeed exist.
What are cathode rays made of?
In 1897, an English physicist J.J. Thomson showed that cathode rays were composed of previously unknown negatively charged particles , which he calculated must have bodies much smaller than atoms and a very large value for their charge-to-mass ratio. He showed that these charged particles could be obtained from just about any material. For example, by the action of ultra-violet light on metals, by heating metal wires or by the ionising action of X-rays. Thomson originally called these particles “ corpuscles ,” and the name electron was proposed for these particles by the Irish physicist George Johnstone Stoney. It was evident that electrons are a component of all neutral atoms, the counterbalancing positive charge being carried by one or more of their other constituent parts. Thomson thus concluded that atoms are divisible, and that the corpuscles are their building blocks.
What is the mass of an electron?
Thompson won the Nobel Prize in physics for establishing the existence of electrons. According to current state of knowledge, the electrons are negatively charged (-1e), almost massless particles that nevertheless account for most of the size of the atom. Their rest mass equal to 9.109 × 10 −31 kg ( 510.998 keV/c2) (approximately 1/1836 that of the proton). Electrons are located in an electron cloud, which is the area surrounding the nucleus of the atom.
Who proposed the Plum pudding model?
But the original idea was different. The original model of an atom, based on the discovery of the electron, was proposed by J. J. Thomson in 1904 and is known as the Plum pudding model or the Thomson model of the atom. It must be noted, this model was proposed before the discovery of the atomic nucleus.
What is an Electron?
The electron is a negatively charged, low-mass particle. As a result, passing close to other electrons or the positive nucleus of an atom might readily deflect it. The first basic particle identified was the electron.
What did Thomson find about cathode rays?
Thomson also installed two magnets on either side of the tube and noticed that the cathode ray was diverted by the magnetic field . Thomson used the findings of these tests to calculate the mass-to-charge ratio of cathode ray particles, which led to a surprising discovery: each particle’s mass was much, much lower than any known atom. Thomson continued his tests with several metals as electrode materials and discovered that the characteristics of the cathode ray were consistent regardless of the cathode material.
What was Thomson's discovery?
The discovery of the electron contradicted Dalton’s atomic theory’s assumption that atoms were indivisible. An altogether new atomic model was required to account for the presence of electrons.
Why are cathode ray tubes called cathode ray tubes?
Because the particle beam, or cathode ray, starts at the cathode, the tubes are termed cathode ray tubes. The beam may be detected by painting phosphors on the tube’s far end, beyond the anode. When the cathode ray strikes the phosphors, they spark or emit light. Thomson surrounded the cathode ray with two oppositely charged electric plates to investigate the particles’ characteristics. The cathode ray was redirected from the negatively charged electric plate to the positively charged plate. The cathode ray was made up of negatively charged particles, according to this.
What particles make up the cathode ray?
Negatively charged particles make up the cathode ray.
What is the first particle to be identified?
The electron is a negatively charged, low-mass particle. As a result, passing close to other electrons or the positive nucleus of an atom might readily deflect it. The first basic particle identified was the electron .
Which subatomic particle was discovered first?
Sir J. J. Thomson first proved the existence of negatively charged particles within an atom called electrons. Thus, the electron is the first sub-atomic particle to be discovered and this paved the way for the discovery of all other sub-atomic particles (i.e. proton and neutron) and also the actual structure of the atom.
What is the force of attraction between negatively charged electrons and positively charged protons?
The force of attraction between negatively charged electrons and positively charged protons helps electrons move in a particular orbit around the nucleus.
Why did Thomson use magnets?
Thomson then placed two magnets on either side of the plates to study the behavior of cathode rays in the presence of a magnetic field. His observation was that the presence of a magnetic field also deflected the cathode rays. The extent of deflection helped him determine the mass to charge ratio. The mass of cathode ray particles was much smaller than the mass of the atom.
What are the fundamental particles of an atom that cannot be further divided?
Unlike protons or neutrons, electrons are the only fundamental particles of an atom that cannot be further divided. They are represented by e or e -.
What is the name of the subatomic particles that revolve around the nucleus of a cell?
Electrons are subatomic particles that are negatively charged and revolve around the nucleus of a cell. JJ Thomson discovered the electrons based on his experiments performed in the discharge tube. This discovery proved that atoms have a complex structure.
What is the nucleus of an atom?
Answer: The nucleus is a dense but small area at the centre of an atom composed of positively charged particles called protons and electrically neutral particles called neutrons. Ernest Rutherford is credited with the discovery of the nucleus.
Why are cathode rays called cathode rays?
This was due to the movement of charge from a negative plate called the cathode to a positive plate called the anode. The rays were termed cathode rays as they were emerging from the cathode plate.
Which experiment led to the discovery of negatively charged particles called electrons?
Answer: The cathode ray discharge tube experiment performed by J.J. Thomson led to the discovery of negatively charged particles called electrons.
What is the Thomson theory?
He is known for the Thomson atomic theory. Many scientists studied the electric discharge of a cat hode ray tube. It was Thomson's interpretation that was important. He took the deflection of the rays by the magnets and charged plates as evidence of "bodies much smaller than atoms.". Thomson calculated these bodies had a large charge-to-mass ratio ...
Why is Thomson's model important?
Yet, Thomson's model is important because it introduced the notion that an atom consisted of charged particles.
What did Thomson's work help explain?
Thomson was closely aligned with chemists of the time. His atomic theory helped explain atomic bonding and the structure of molecules.
What was the smallest unit of matter?
Prior to Thomson's discovery of electrons, scientists believed the atom was the smallest fundamental unit of matter.
When was the atom thought to be a solid sphere?
Up until the end of the 19th century, atoms were thought to be tiny solid spheres. In 1903, Thomson proposed a model of the atom consisting of positive and negative charges, present in equal amounts so that an atom would be electrically neutral.
Who proposed the atom as a sphere of positive matter with electrons positioned based on electrostatic forces?
Thomson calculated these bodies had a large charge-to-mass ratio and he estimated the value of the charge itself. In 1904, Thomson proposed a model of the atom as a sphere of positive matter with electrons positioned based on electrostatic forces.
When was the discovery of potassium?
Thomson discovered the natural radioactivity of potassium in 1905. In 1906, Thomson demonstrated a hydrogen atom had only a single electron. Thomson's father intended for J.J. to be an engineer, but the family did not have the funds to support the apprenticeship.
Who was the first person to discover the electron?
Sir Joseph John Thompson OM PRS (18 December 1856 – 30 August 1940) was a British physicist and Nobel Laureate in Physics, credited with the discovery of the electron, the first subatomic particle to be discovered.
What did Thomson discover about cathode rays?
In 1897, Thomson showed that cathode rays were composed of previously unknown negatively charged particles (now called electrons), which he calculated must have bodies much smaller than atoms and a very large charge-to-mass ratio. Thomson is also credited with finding the first evidence for isotopes of a stable (non-radioactive) element in 1913, as part of his exploration into the composition of canal rays (positive ions). His experiments to determine the nature of positively charged particles, with Francis William Aston, were the first use of mass spectrometry and led to the development of the mass spectrograph.
How did Thomson find the path of cathode rays?
Thomson detected their path by the fluorescence on a squared screen in the jar.
How did Thomson find the charge to mass ratio?
By comparing the deflection of a beam of cathode rays by electric and magnetic fields he obtained more robust measurements of the mass-to-charge ratio that confirmed his previous estimates. This became the classic means of measuring the charge-to-mass ratio of the electron. (The charge itself was not measured until Robert A. Millikan 's oil drop experiment in 1909.)
Why did Thomson believe their experiments were flawed?
Previous experimenters had failed to observe this, but Thomson believed their experiments were flawed because their tubes contained too much gas. Thomson constructed a Crookes tube with a better vacuum.
How many children did George Paget Thomson have?
They had two children: George Paget Thomson, who was also awarded a Nobel Prize for his work on the wave properties of the electron, and Joan Paget Thomson (later Charnock), who became an author, writing children's books, non-fiction and biographies.
What was William Thomson's work on the motion of vortex rings?
Thomson's prize-winning master's work, Treatise on the motion of vortex rings, shows his early interest in atomic structure. In it, Thomson mathematically described the motions of William Thomson 's vortex theory of atoms.
