
When did John Dalton invent the first atomic theory?
John Dalton discovered the atomic theory of matter in 1803. During his studies in meteorology, Dalton found out that water exists in air as an independent gas. Dalton thought about how air and water could occupy the same place at the same time, when solid's can't. Dalton thought that evaporation might be the mixing of air and water particles.
How did John Dalton make the atomic theory?
How did John Dalton discover the atomic theory? In 1803 Dalton discovered that oxygen combined with either one or two volumes of nitric oxide in closed vessels over water and this pioneering observation of integral multiple proportions provided important experimental evidence for his incipient atomic ideas.
What year John Dalton discovered atomic theory?
However, a recent study of Dalton’s laboratory notebook entries concludes he developed the chemical atomic theory in 1803 to reconcile Henry Cavendish ’s and Antoine Lavoisier ’s analytical data on the composition of nitric acid, not to explain the solubility of gases in water.
What year did John Dalton propose his atomic model?
John Dalton, an English schoolteacher was responsible for proposing his atomic theory in 1808. Using the idea that elements are composed of atoms, Dalton developed his theory as an explanation for the law of conservation of mass, the law of definite proportions, and the law of multiple proportions.
See more
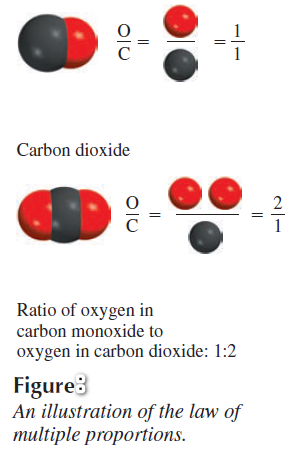
Was Dalton's theory accepted?
John Dalton's atomic theory was generally accepted because it explained the laws of conservation of mass, definite proportions, multiple proportions, and other observations. Although exceptions to Dalton 's theory are now known, his theory has endured reasonably well, with modifications, throughout the years.
When was Dalton's atomic theory proven wrong?
These atoms of different masses are called isotopes. For example, chlorine has two isotopes with mass numbers 35 and 37. Dalton also claimed that atoms of different elements are different in all respects. This has been proven wrong in certain cases: argon and calcium atoms each have an atomic mass of 40 amu.
Is Dalton's atomic theory still accepted today?
Dalton's atomic theory was accepted by many scientists almost immediately. Most of it is still accepted today. However, scientists now know that atoms are not the smallest particles of matter. Atoms consist of several types of smaller particles, including protons, neutrons, and electrons.
Who first disproved Dalton's atomic theory?
Atomic Theory. In 1897, English physicist J. J. Thomson (1856–1940) disproved Dalton's idea that atoms are indivisible. When elements were excited by an electrical current, atoms break down into two parts. One of those parts is a negative tiny particle, which Thomson called a corpuscle in 1881.
Why was Dalton's model disproved?
It does not account for subatomic particles: Dalton's atomic theory stated that atoms were indivisible. However, the discovery of subatomic particles (such as protons, electrons, and neutrons) disproved this postulate.
Why did Dalton's atomic theory failed?
Dalton's atomic theory suggested that the atom was indivisible and indestructible. But the discovery of two fundamental particles (electrons and protons) inside the atom, led to the failure of this aspect of Daltons atomic theory.
Which atomic theory is currently accepted?
The electron cloud model is currently the most sophisticated and widely accepted model of the atom. It retains the concept of the nucleus from Bohr and Rutherford's models, but introduces a different definition of the motion of electrons around the nucleus.
What experiment disproved Dalton's theory?
While controversial at first, Thomson's discoveries were gradually accepted by scientists. Eventually, his cathode ray particles were given a more familiar name: electrons. The discovery of the electron disproved the part of Dalton's atomic theory that assumed atoms were indivisible.
Who first discovered atom?
John Dalton (1766-1844), a great chemist, really started the modern atomic hypothesis. His atom however was like a solid billiard ball.
Who proved the existence of atoms?
In 1808, chemist John Dalton developed a very persuasive argument that led to an amazing realization: Perhaps all matter (i.e., stuff, things, objects) is made of tiny, little bits.
Which postulate S of Dalton's atomic theory of matter is are not true?
'Atoms of a single element can have different masses' is not present in postulates of Dalton's atomic theory. It is in fact a limitation of Dalton's atomic theory. Hence, option D is the correct choice.
Which of Dalton's theories are considered correct today?
Although two centuries old, Dalton's atomic theory remains valid in modern chemical thought. 1) All matter is made of atoms. Atoms are indivisible and indestructible. 3) Compounds are formed by a combination of two or more different kinds of atoms.
Who incorrectly theorized that atoms were indivisible and that all atoms of a given element?
Democritus developed his atomic philosophy as a middle ground between two opposing Greek theories about reality and the illusion of change. He argued that matter was subdivided into indivisible and immutable particles that created the appearance of change when they joined and separated from others.
How did JJ Thomson's experiment change Dalton's picture of the atom?
Explanation: Thomson's experiments with cathode ray tubes helped him to discover the electron (which Dalton did not know about). Dalton thought that atoms were indivisible particles, and Thomson's discovery of the electron proved the existence of subatomic particles.
How does Dalton’s atomic theory explain the law of conservation of mass?
Since it states that atoms cannot be created or destroyed, Dalton’s theory suggests that the net mass of the participating species in a chemical re...
How does Dalton’s atomic theory differentiate between elements and compounds?
This theory states that elements combine in fixed, whole-number ratios to form compounds. Therefore, it suggests that compounds are made up of mole...
What are the 5 key postulates of Dalton’s atomic theory?
The 5 postulates of Dalton’s atomic theory are listed below. All matter is made up of atoms, which are tiny, indivisible particles. All the atoms o...
List two merits of Dalton’s atomic theory.
One of the most important merits of Dalton’s atomic theory is the fact that the theory does not violate several fundamental laws of chemical combin...
What are the shortcomings of Dalton’s atomic theory?
Some important demerits of Dalton’s atomic theory are listed below. The theory did not account for the existence of subatomic particles (it suggest...
Do electrons actually exist?
Most of us realize that the neutron, in an atom of matter, is a negatively charged particle orbiting the nucleus. No two electrons at the same time...
Which atomic model is used today?
The Bohr paradigm, generally speaking, encapsulates the popular understanding of the atom. In artwork that depicts a central atomic nucleus and ova...
Why can’t you see an atom with the naked eye?
We can not see an atom with naked eyes because an atom is extremely small and is not perceptible.
Can atoms be divided or destroyed?
No, atoms can not be divided or destroyed. However, it can combine with other atoms to form compounds. In a chemical reaction, an atom can combine,...
1. What are the Laws Supporting Dalton’s Atomic Theory?
Dalton’s atomic model was mainly based on two laws. The two laws are: Law of Conservation of Mass: The law of conservation of mass states that mass...
2. What are the Merits of Dalton’s Atomic Theory?
Despite its various shortcomings, Dalton’s atomic theory explained a lot and provided a framework for the modern atomic theory.It explained that ma...
3. What is Rutherford’s Atomic Model and What were its Limitations?
Ernest Rutherford was a British scientist who conducted an experiment and proposed the atomic structure of different elements. This was called the...
4. What are Isotopes?
The total components of the nucleus of an atom are called nucleons. A nucleon can consist of either a proton or a neutron. All elements have a uniq...
5. What was Bohr’s Atomic Model and What were its Limitations?
Neils Bohr was a scientist who proposed his atom model in 1915, which became popular. Nucleons are the constituents of an atom's nucleus. A proton...
What are the merits of Dalton's atomic theory?
One of the most important merits of Dalton’s atomic theory is the fact that the theory does not violate several fundamental laws of chemical combination such as the law of definite proportions, the law of multiple proportions, and the law of conservation of mass. Another important merit of Dalton’s atomic theory is that it provided a basis ...
How does Dalton’s atomic theory differentiate between elements and compounds?
This theory states that elements combine in fixed, whole-number ratios to form compounds. Therefore, it suggests that compounds are made up of molecules that contain two or more atoms of different elements.
What is the name of the theory that states that matter is made up of atoms?
Dalton’s Atomic Theory. Dalton’s atomic theory was a scientific theory on the nature of matter put forward by the English physicist and chemist John Dalton in the year 1808. It stated that all matter was made up of small, indivisible particles known as ‘atoms’. All substances, according to Dalton’s atomic theory , are made up of atoms, ...
Which theory failed to explain the dissimilarities in the properties of different allotropes of an element?
Dalton’s atomic theory failed to explain the dissimilarities in the properties of different allotropes of an element. This theory states that elements must combine in simple, whole-number ratios to form compounds. However, this is not necessarily true.
What does Dalton's theory say about the law of conservation of mass?
Since it states that atoms cannot be created or destroyed, Dalton’s theory suggests that the net mass of the participating species in a chemical reaction is conserved. This postulate, therefore, accounts for the law of conservation of mass.
Does Dalton's atomic theory account for subatomic particles?
It does not account for subatomic particles: Dalton’s atomic theory stated that atoms were indivisible. However, the discovery of subatomic particles (such as protons, electrons, and neutrons) disproved this postulate. It does not account for isotopes: As per Dalton’s atomic theory, all atoms of an element have identical masses and densities.
Can atoms be destroyed?
However, atoms of different element exhibit different properties and vary in mass and size. Atoms can neither be created nor destroyed. Furthermore, atoms cannot be divided into smaller particles. Atoms of different elements can combine with each other in fixed whole-number ratios in order to form compounds.
What is the basis of Dalton's atomic theory?
The law of conservation of mass and constant proportions are the basis that helps explain Dalton’s atomic theory. Based on these laws Dalton’s atomic theory states the following postulates:
What did Dalton contribute to the atomic theory?
Dalton’s atomic theory contributed a lot to modern atomic theory. Dalton’s model was revolutionary for the period and gave much to the new chemists to research upon. The Atomic theory got modified with the contribution of many after Dalton, namely, Chadwick, JJ Thompson, Ernest Rutherford, Niels Bohr, etc.
How do atoms form compounds?
Atoms of different elements combine in whole numbers in a simple but fixed ratio to form compounds. Different types of atoms are joined together to form compounds. A chemical reaction is a rearrangement of atoms, where the formation of new products occurs due to the rearrangement of atoms in the reactant.
What is the theory of Dalton?
The theory of Dalton was published in the paper “New Chemical Philosophy”. Dalton’s idea for the theory is believe d to be inspired by the physical properties of gases. Although connections of his work have been made with several other chemists of the time.
Who came up with the atomic model?
Dalton ’s Atomic Model. The matter has been a subject of fascination since the beginning times. Democritus was known to be the first to suggest that matter is made up of particles. Dalton’s model came almost two millennia later and brought further light to the topic.
Who was the first scientist to study the atom?
Dalton’s Early Model of The Atom. The introduction to the early theory of the atom was done by a scientist named John Dalton (1766-1844). He was a British physicist, chemist, and meteorologist who is well known for many of his contributions to the pioneering research of atoms, the law of partial pressures, Daltonism, etc.
Does the theory of allotropes explain the difference in properties?
The theory fails to explain the difference in properties of allotropes. It can not prove the difference in properties of charcoal, diamond, and graphite; allotropes of carbon.
What are the two laws that Dalton formulated?
Dalton formulated his theory based on two laws: the law of conservation of mass and the law of constant composition.
What is the atomic theory of matter?
According to Dalton’s atomic theory, all substances are made up of atoms, which are indestructible and indivisible building blocks. While the atoms of one element were all the same size and mass, other elements had atoms of different sizes and weights.
What is a compound in atomic theory?
Compounds are combinations of two or more different types of atoms: In the third part of Dalton’s atomic theory, he proposed that compounds are combinations of two or more different types of atoms. An example of such a compound is Common Salt. Common Salt is a combination of two different types of elements with varying physical and chemical properties. The first, sodium, is a highly reactive metal. The second, chlorine, is a toxic gas. When they react, the atoms combine in a 1:1 ratio to form white crystals of NaCl.
Who was the first person to believe that matter is made up of particles?
Democritus is credited as being the first to postulate that matter is made up of particles. These particles were given the name atomos, which means indivisible in Greek. Democritus ’ Atomic Theory was based on this. Scientists had very little information on this idea at the time due to a lack of technical setup.
Who proposed the idea of simplifying matter?
Scientist John Dalton manifested the works on simplifying matter over two thousand years later. John Dalton proposed the famous Dalton’s Atomic Theory in 1808. In a paper titled “A New Chemical Philosophy,” he published this idea; certainly, the philosophy was novel at the time. Let’s have a look at the theory’s postulates.
Does Dalton's atomic theory violate the law of multiple proportions?
Dalton’s atomic theory. doesn’t violate the law of multiple proportions, the law of conservation of mass, and the law of constant proportions.
Can a molecule be formed from a single atom?
In that sense, no, by nature, a molecule can not be formed from a single atom.
Why was Dalton's theory widely accepted?
Dalton's theory became widely accepted because it was based on quantitative experimental data, rather than pure qualitative observations.
Is an atom indivisible?
2. Atoms are indivisible. During a chemical reaction, atoms can be rearranged. However, they are never created nor distroyed.
What is the significance of Dalton's atomic theory?
Merits of Dalton's Atomic Theory. The atomic theory explains the laws of chemical combination (the Law of Constant Composition and the Law of Multiple Proportions ). Dalton was the first person to recognize a workable distinction between the fundamental particle of an element (atom) and that of a compound (molecule).
Who was the first person to publish his theory of atoms?
Postulates of Dalton's Atomic Theory. John Dalton, a British school teacher, published his theory about atoms in 1808. His findings were based on experiments and the laws of chemical combination.
Who is the author of the atom theory?
Merits of Dalton's Atomic Theory. Contributors and Attributions. John Dalton, a British school teacher, published his theory about atoms in 1808. His findings were based on experiments and the laws of chemical combination.
How do atoms of different elements combine?
Atoms of different elements may combine with each other in a fixed, simple, whole number ratios to form compound atoms. Atoms of same element can combine in more than one ratio to form two or more compounds. The atom is the smallest unit of matter that can take part in a chemical reaction.
Which periodic table had how many blank spaces left in it which represented elements that had not yet been discovered?
Mendeleev's periodic table had how many blank spaces left in it which represented elements that had not yet been discovered
What are atoms with the same number of protons and a different number of neutrons called?
atoms with the same number of protons and a different numbers of neutrons are called isotopes
Who noticed patterns when he arranged the elements in what way?
Mendeleev noticed that patterns appeared when he arranged the elements in what way
What does it mean when an atom is neutral?
when an atom is electrically neutral it means that the overall charge of an atom is zero

History of Dalton Atomic Theory
- Alchemy, an ancient science, existed till the late 16thcentury. It was a series of scientific advancements in the field of science. At that time, the chemistry was not even considered a science. It was because alchemy was a spiritual and magical essence, whereas chemistry related to structures and compositions of materials. The works of Robert Boyl...
Postulates of John Dalton’s Atomic Theory
- There are five basic postulates of Dalton’s atomic theory. 1. Each element is made up of tiny and indestructible particles called atoms. 2. Atoms of a single element are always identical. 3. Atoms of different elements are different in terms of their atomic weights and chemical properties. 4. Chemical compounds are formed by the combination of atoms of different elements in specific ra…
Explanation of Postulates
- Dalton worked and expanded the work of Leucippus of Miletus (5th-century B.C.E) who assumed that all substances are made up of solid, hard, impenetrable, and mobile particles, which he called “atomos” (literally indivisible) in around 430 B.C.E. Dalton proposed that every single atom of an element is the same as other atoms of that element. For example, all the atoms present in zinc a…
Limitation of Dalton’s Atomic Theory
- Although Dalton’s atomic theory can be regarded as a major step in the development of the first complete atomic theory in terms of atoms and their properties, it also had some shortcomings. These limitations got picked up by the working of modern scientists like; J.J Thompson, Ernest Rutherford, Neil Bohr, etc. 1. Dalton’s atomic theory could not explain why elements have differe…
Key Takeaway
- Dalton assumed that one atom of hydrogen and oxygen each when combined, constitute water. Through this and his further studies, he hypothesized that an oxygen atom is 5.6 times heavier than a hydro...
- He was the one to realize that new products (compounds) are actually created by the rearrangement of individual atoms.
- Dalton assumed that one atom of hydrogen and oxygen each when combined, constitute water. Through this and his further studies, he hypothesized that an oxygen atom is 5.6 times heavier than a hydro...
- He was the one to realize that new products (compounds) are actually created by the rearrangement of individual atoms.
- There were many elements, that, for the first time, were revealed as compounds by Dalton.
Concepts Berg
- What are the Merits of Dalton’s Atomic Theory? Merits of Dalton’s atomic theory: 1. It approves the law of conservation of mass and the law of proportions. 2. It explains the difference between atoms, elements, and compounds. 3. It provided the complete basic framework for the development of new and advanced theories of atoms. How does Dalton’s atomic theory different…