What is an example of a Hydrogenous sediment?
Due to ocean water precipitation or ion exchange between ocean water and sediments, hydrogenous sediment forms. Examples include metal sulfides, evaporites, and manganese nodules. This type of sediment comes from dissolved material in water.
Where do biogenous sediments come from?
Biogenous sediments come from organisms like plankton when their exoskeletons break down. Hydrogenous sediments come from chemical reactions in the water. Cosmogenous sediments come from space, filtering in through the atmosphere or carried to Earth on meteorites. What is the most common Biogenous sediment? Contents
Why is there so little Hydrogenous sediment in the ocean?
These reactions are usually triggered by a change in conditions, such as a change in temperature, pressure, or pH, which reduces the amount of a substance that can remain in a dissolved state. There is not a lot of hydrogenous sediment in the ocean compared to or sediments, but there are some interesting forms. were discussed in section 4.11.
What are the components of hydrogenous rocks?
Besides evaporates, hydrogenous components include hydrothermal deposits, metalliferous elements, such as polymetallic nodules and crusts, and a variety of authigenic minerals.
How are sediments formed?
What type of sediment is molten rock?
What is a methane hydrate?
What is methane in seafloor sediments?
Where are manganese nodules found?
Where is the oldest salt produced?
What causes a substance to precipitate out as a solid?
See 2 more
Where does Hydrogenous sediment come from?
Hydrogenous sediments come from chemical reactions in the water. Cosmogenous sediments come from space, filtering in through the atmosphere or carried to Earth on meteorites.
How does a hydrogenous sediment form in the deep ocean?
Evaporites are hydrogenous sediments that form when seawater evaporates, leaving the dissolved materials to precipitate into solids, particularly halite (salt, NaCl). In fact, the evaporation of seawater is the oldest form of salt production for human use, and is still carried out today.
What are examples of hydrogenous sediments?
Hydrogenous sediments are sediments directly precipitated from water. Examples include rocks called evaporites formed by the evaporation of salt bearing water (seawater or briny freshwater).
Where are the sediments found?
Deltas, river banks, and the bottom of waterfalls are common areas where sediment accumulates. Glaciers can freeze sediment and then deposit it elsewhere as the ice carves its way through the landscape or melts.
What type of sediment is found in the deep ocean?
Most deep ocean sediments are silt and mud. Most sediments form as rocks are broken down into smaller particles such as sand and clay.
What type of sediments are found in the ocean?
We classify marine sediments by their source. The four main types of sediment are lithogenous, biogenous, hydrogenous and cosmogenous (Table 1 below). In this lab, you will primarily examine lithogenous, biogenous, and hydrogenous sediments.
What is Hydrogenous sediment made of?
3) Hydrogenous Sediments: These are minerals that have directly precipitated from seawater, such as sulfides from hydrothermal vents, materials leaching out of the MOR, or stuff from the continents in river runoff.
What are the three types of hydrogenous sediments?
Some hydrogenous sediments include halite (salt), chemical limestone and manganese nodules.
What are the 3 types of ocean floor sediments?
There are three kinds of sea floor sediment: terrigenous, pelagic, and hydrogenous. Terrigenous sediment is derived from land and usually deposited on the continental shelf, continental rise, and abyssal plain.
What type of sediment is typically found in lake bottoms?
clayAnswer and Explanation: In lake bottoms, the most common form of sediment is clay, which is the finest (smallest) particle size of clastic sediments. Clastic sediments are organized by grain size, ranging in descending order from boulder to cobble, pebble, sand, silt, and finally clay.
Which sediment type is the least common in the ocean and why?
Siliceous ooze Siliceous oozes are the least common of the deep sea sediments, and make up approximately 15% of the ocean floor. Oozes are defined as sediments which contain at least 30% skeletal remains of pelagic microorganisms.
Where are most sediments and sedimentary rocks found?
Most sediments and sedimentary rocks are found at the bottom or near the edges of bodies of water like lakes, rivers, and oceans. This is because bodies of water often help to weather existing rock, creating sediment particles.
How does a hydrogenous sediment form in the deep ocean quizlet?
Most hydrogenous sediments originate from chemical reactions that occur on particles of the dominant sediment. The most famous hydrogenous sediments are manganese nodules.
Where does sediment on the bottom of the ocean come from?
Sediment on the seafloor originates from a variety of sources, including biota from the overlying ocean water, eroded material from land transported to the ocean by rivers or wind, ash from volcanoes, and chemical precipitates derived directly from seawater.
What type of hydrogenous sediment is formed quickly at hydrothermal vents?
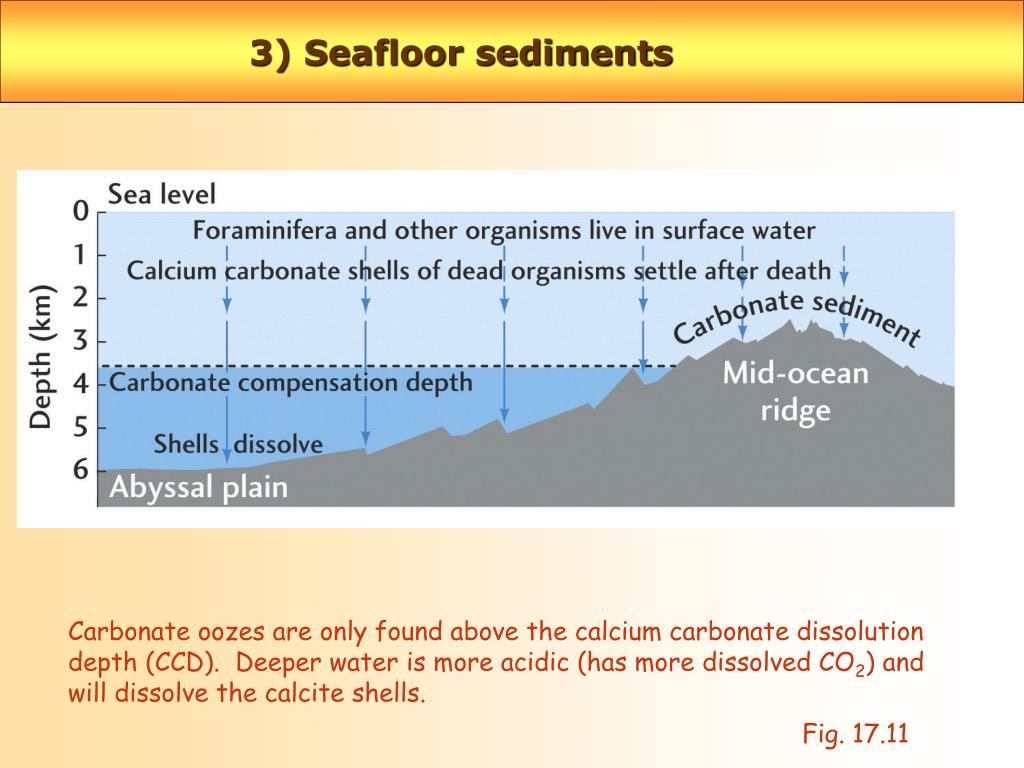
Hydrogenous Sediments Calcium carbonates - form when CaCO3 enriched water precipitates out calcite, which accumulates on the sea floor to form layers. These sediments lithify the CaCO3 mud into LIMESTONE. Metal sulfides - form in hydrothermal vent systems.
What is Hydrogenous sediment made of?
3) Hydrogenous Sediments: These are minerals that have directly precipitated from seawater, such as sulfides from hydrothermal vents, materials leaching out of the MOR, or stuff from the continents in river runoff.
What is hydrogenous sediment?
Due to ocean water precipitation or ion exchange between ocean water and sediments, hydrogenous sediment forms. Examples include metal sulfides, evaporites, and manganese nodules. This type of sediment comes from dissolved material in water. Within seawater, minerals precipitate out of solution due to chemical reactions.
Where do phosphorus-bearing compounds occur?
Phosphates often occur as nodules on the continental shelf and rock coatings. They are phosphorous-bearing compounds and accurately indicate where there is a high concentration of biological activity. Calcite and aragonite, consisting of calcium carbonate, are the most important carbonate minerals in marine sediment.
Where do evaporite minerals accumulate?
Evaporite minerals accumulate where open ocean circulation is highly restricted and where evaporation frequently occurs. Dissolved minerals become saturated with seawater left from evaporation and later starts to precipitate. They either sink or form crusts of evaporite minerals.
Where are manganese nodules found?
They are commonly found on deep-ocean floors , and their major components are iron oxide (20%) and manganese dioxide (30%). Scientists remain unsure about how manganese nodules contain such high concentrations of manganese when they precipitate from seawater.
Is carbonate a biogene?
Although the majority of carbonate deposits are biogenous, hydrogenous carbonate deposits can sometimes precipitate from seawater and produce aragon ite crystals. Metal sulfide deposits are strongly connected with black smokers and hydrothermal vents along the mid-ocean ridge.
What are the components of hydrogenous sediments?
Besides evaporates, hydrogenous components include hydrothermal deposits, metalliferous elements, such as polymetallic nodules and crusts, and a variety of authigenic minerals. This chapter focuses on metalliferous and authigenic deposits, which have not been described before and are either widespread in the ocean or characteristic of specific environmental conditions. They include polymetallic nodules and crusts, glauconite, and phosphates. First recovered from the deep ocean during the Challenger Expedition, polymetallic nodules are mainly concentrated on the seafloor of the deepest basins, whereas polymetallic crusts are principally associated to submarine volcanic systems and seamounts. Overall, there is a strong relationship between seawater and the geochemical composition of many metalliferous deposits. Although chemical elements of hydrothermal origin largely dominate, local environmental conditions may also play a role in the elemental content of metalliferous deposits. For example, polymetallic crusts and nodules may include elements of biogenic origin (Ba, Ca, P, etc.) or detrital origin (Si, Al, etc.).
Which crusts increase in importance with distance from the hydrothermal source area?
Hydrogenous crusts rapidly increase in importance with distance from the hydrothermal source area. In the central Pacific Ocean, for example, they represent by far the most widespread polymetallic deposits on seamounts and plateaus.
What is birnessite made of?
Birnessite, which is an important component of polymetallic crusts and nodules , and is also found in hydrothermal deposits. Birnessite is generally fine grained and has a relatively poorly organized sheet structure. The sheet units are made of MnO 6 octahedral layers, free valences being filled by exchangeable cations and water in an interlayer position. Exchangeable cations principally include Ni and Ba, but also Co, Na, Ca and Mg. Dehydrated birnessite sometimes occurs in hydrothermal environments, where the presence of exchangeable, interlayered cations protect to a certain extent the mineral structure from collapse.
What is the dominant manganese-bearing phase found in both polymetallic crusts and nodules?
Vernadite, which is the dominant manganese-bearing phase found in both polymetallic crusts and nodules. It is a fine-grained phase of poor crystallinity. Its structure is unclear, but chemical analyses show that vernadite commonly includes minor percentages of Fe, K, Mg, Ca and Ba, as well as water.
What are the elements that are found in metalliferous deposits?
Iron concentrations are generally lower, below 20–25%. Associated elements include copper, zinc, nickel , chromium and lead, but in much smaller proportions generally ranging from a few parts per million to a few percents. Manganese is abundant in seawater, and is predominantly of hydrothermal origin like most associated elements. The predominance of manganese in metalliferous sediments of the deep ocean is a consequence of the rapid incorporation of ore elements such as nickel and zinc into sulfide minerals of hydrothermal deposits (see Section 6.4.2). Then, hydrothermal fluids mix with seawater. Metalliferous elements precipitate in higher proportions in those oceanic regions adjacent to areas of active volcanism and hydrothermalism, i.e., active spreading ridges and regions of intraplate, hot-spot volcanism where seamounts are abundant. Metalliferous elements are also widely dispersed beneath hydrothermal plumes and can participate in the formation of hydrogenous minerals in oceanic regions remote from the source area. However, their accumulation so as to form metalliferous deposits requires very low sedimentation rates of other sediment particles. These conditions are fulfilled in deep oceanic basins of weak terrigenous input, low surface productivity and/or significant dissolution of biogenic and organic elements. As a consequence, metalliferous deposits are more abundant in the deep basins of the Indian Ocean and especially the Pacific Ocean. The relationship between spreading ridge and seamount activity, and the occurrence of metalliferous deposits, suggests that intervals of increased spreading rates (which are also intervals of enhanced intraplate volcanism and seamount activity) may coincide with increased accumulation rates of metalliferous deposits. It is generally admitted that metalliferous oxides and hydroxides accrete at growth rates that range from 0.5–10 mm/Myr, but some estimates may include intervals of non-deposition.
Where are the highest concentrations of metalliferous elements found?
By far, the largest concentrations in metalliferous elements (especially Mn) are found at localities between 1,100 and 2,000 m water depth ( Figure 13.13 ). This does not reflect the degree of anoxia and related remobilization of metals since intermediate-depth sapropels are relatively poor in organic material, highest concentrations being recorded at maximum depths of the basins. It is likely that higher quantities of dissolved metals were in fact released from the deepest sediment into bottom waters during sapropel formation. Dissolved metals migrated within deep, oxygen-depleted waters, where concentrations increased. Precipitation of metal oxides probably occurred preferentially at the transition depth between deep, oxygen-depleted waters and more oxygenated, intermediate waters, the particulate oxides settling onto higher topography being better preserved from further dissolution.
Where are polymetallic nodules found?
Polymetallic nodules are commonly found in the deepest oceanic basins of more than 4,500 m water depth, where very low sedimentation rates of a few millimeters per million years are associated to an efficient bottom circulation. They are however more abundant on abyssal hills and seamounts than in abyssal lows. Polymetallic nodules are concretions of irregular, spheroidal to ellipsoidal shape, which commonly are 20–80 mm in size. Smaller nodules are generally sub-spheroidal, the degree of sphericity decreasing as the nodule size increases. This suggests that nodules grow more rapidly along their horizontal axes, progressively taking a sub-ellipsoidal shape. However, larger nodules sometimes show irregular shapes. The upper surface of the nodules (which is in contact with seawater) may be smooth to finely granulated. In contrast, the lower surface of the nodules (which is in contact with sediments) may be rough and coarsely granulated. The surface texture of polymetallic nodules also varies at regional scale, and nodules may have overall smoother surfaces in some areas compared to others. In the central Indian Ocean, for example, polymetallic nodules in the deepest abyssal plain have a coarser surface texture than those in elevated areas.
Where are coarse sediments less common?
Coarse lithogenous sediments are less common in the central ocean, as these areas are too far from the sources for these sediments to accumulate. Very small particles are the exception, and as described below, they can accumulate in areas that other lithogenous sediment will not reach. The distribution of. biogenous sediments.
How long does it take for sediment to accumulate in the ocean?
Rates of sediment accumulation are relatively slow throughout most of the ocean, in many cases taking thousands of years for any significant deposits to form. accumulates the fastest, on the order of 1 m or more per thousand years for coarser particles.
What percentage of the seafloor is covered by siliceous oozes?
Approximately 15% of the seafloor is covered by siliceous oozes. Biogenous calcium carbonate sediments also require production to exceed dissolution for sediments to accumulate, but the processes involved are a little different than for silica. Calcium carbonate dissolves more readily in more acidic water.
Why are sediments thinner?
is being formed, sediments are thinner, as they have had less time to accumulate on the younger crust. As you move away from the ridge spreading center the sediments get progressively thicker (see section 4. 5), increasing by approximately 100-200 m of sediment for every 1000 km distance from the ridge axis.
Where do clays dominate?
Clays dominate in the central North Pacific, for example. This area is too far from land for coarse lithogenous sediment to reach, it is not productive enough for biogenous tests to accumulate, and it is too deep for calcareous materials to reach the bottom before dissolving.
Is biogenous sediment more abundant in coastal areas?
So coastal areas remain dominated by lithogenous sediment, and biogenous sediments will be more abundant in environments where there is little lithogenous input. In order for biogenous sediments to accumulate their rate of production must be greater than the rate at which the tests dissolve.
How are sediments formed?
sediments formed from the precipitation of dissolved substances (12.4) sediment derived from preexisting rock (12.2) sediment created from the remains of organisms (12.3) area of the seafloor where superheated water seeps out of the crust (4.11) molten rock typically dominated by silica (3.2)
What type of sediment is molten rock?
molten rock typically dominated by silica (3.2) spherical accumulations of manganese and other metals that form slowly through precipitation on the seafloor (12.4) hydrogenous sediments that form when seawater evaporates (12.4)
What is a methane hydrate?
Methane hydrates. are another type of hydrogenous deposit with a potential industrial application. All terrestrial erosion products include a small proportion of organic matter derived mostly from terrestrial plants. Tiny fragments of this material plus other organic matter from marine plants and animals accumulate in.
What is methane in seafloor sediments?
The methane within seafloor sediments represents an enormous reservoir of fossil fuel energy. Although energy corporations and governments are anxious to develop ways to produce and sell this methane, anyone that understands the climate-change implications of its extraction and use can see that this would be folly.
Where are manganese nodules found?
Therefore, manganese nodules are usually limited to areas in the central ocean, far from significant lithogenous or biogenous inputs, where they can sometimes accumulate in large numbers on the seafloor (Figure 12.4.2 right ).
Where is the oldest salt produced?
In fact, the evaporation of seawater is the oldest form of salt production for human use, and is still carried out today. Large deposits of halite evaporites exist in a number of places, including under the Mediterranean Sea.
What causes a substance to precipitate out as a solid?
Occasionally chemical reactions occur that cause these substances to precipitate out as solid particles, which then accumulate as. hydrogenous sediment. . These reactions are usually triggered by a change in conditions, such as a change in temperature, pressure, or pH, which reduces the amount of a substance that can remain in a dissolved state.
