
What is the function of protease?
( PDB: 1LVB ) A protease (also called a peptidase or proteinase) is an enzyme that catalyzes (increases the rate of) proteolysis, the breakdown of proteins into smaller polypeptides or single amino acids. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds.
What are the proteases secreted from the stomach?
Acid proteases secreted into the stomach (such as pepsin) and serine proteases present in the duodenum ( trypsin and chymotrypsin) enable us to digest the protein in food.
Which proteases are used to cleave fusion proteins?
Highly specific proteases such as TEV protease and thrombin are commonly used to cleave fusion proteins and affinity tags in a controlled fashion. The activity of proteases is inhibited by protease inhibitors.
How are proteases classified by their pH?
Alternatively, proteases may be classified by the optimal pH in which they are active: Neutral proteases involved in type 1 hypersensitivity. Here, it is released by mast cells and causes activation of complement and kinins. This group includes the calpains.
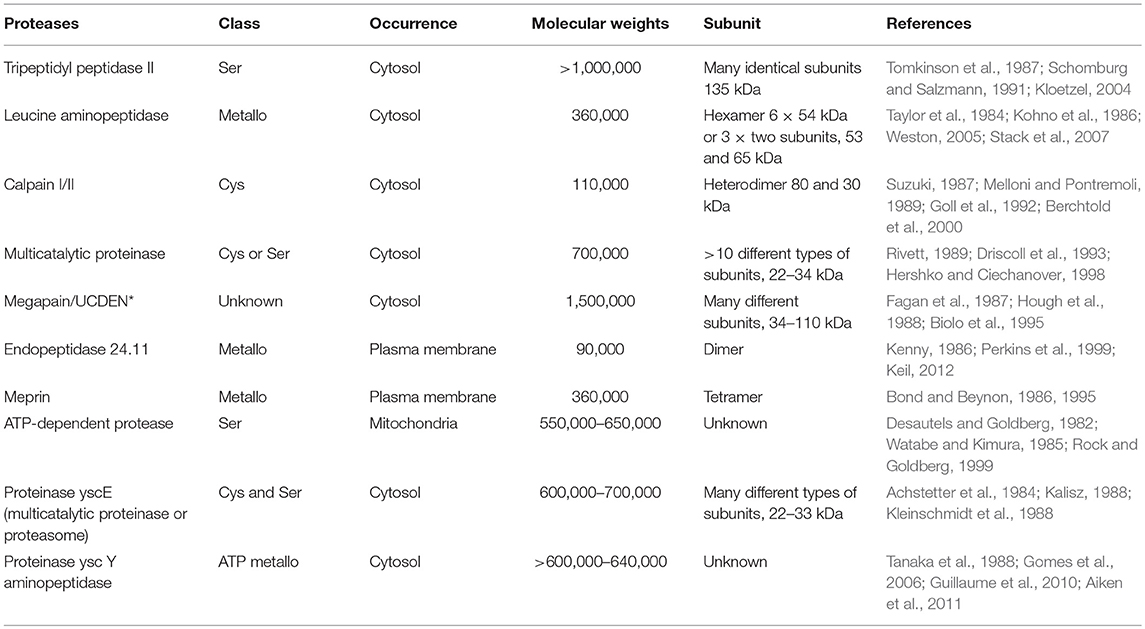
Where are protease enzymes stored?
Protease. Protease is produced in the stomach, pancreas, and small intestine. Most of the chemical reactions occur in the stomach and small intestine.
Where is protease found and what does it do?
Proteases are used throughout an organism for various metabolic processes. Acid proteases secreted into the stomach (such as pepsin) and serine proteases present in the duodenum (trypsin and chymotrypsin) enable us to digest the protein in food. Proteases present in blood serum (thrombin, plasmin, Hageman factor, etc.)
What are proteases found in?
Proteases are found in animals, plants, bacteria, archaea, and viruses. Proteases are involved in protein processing, regulation of protein function, apoptosis, viral pathogenesis, digestion, photosynthesis, and numerous other vital processes.
Where is protease released from?
the pancreasProteases Several proteases are synthesized in the pancreas and secreted into the lumen of the small intestine.
What is the function of proteases?
Proteolytic enzymes (proteases) are enzymes that break down protein. These enzymes are made by animals, plants, fungi, and bacteria. Proteolytic enzymes break down proteins in the body or on the skin. This might help with digestion or with the breakdown of proteins involved in swelling and pain.
What does protease break down into?
proteolytic enzyme, also called protease, proteinase, or peptidase, any of a group of enzymes that break the long chainlike molecules of proteins into shorter fragments (peptides) and eventually into their components, amino acids.
Is protease found in the mouth?
Whole human saliva contains a number of proteolytic enzymes, mostly derived from white blood cells and bacteria in the oral cavity. However, less information is available regarding proteases produced by salivary glands and present in salivary secretions.
What form of protease is released in the stomach?
Specific cells within the gastric lining, known as chief cells, release pepsin in an inactive form, or zymogen form, called pepsinogen. By doing so, the stomach prevents the auto-digestion of protective proteins in the lining of the digestive tract.
What is the function of a protease quizlet?
Function: Protease breaks down proteins. The break down the peptide bonds in protein foods to liberate the amino acids needed by the body. Example of a protease is pepsin, which is found in the stomach.
What is protease in biology?
proteolytic enzyme, also called protease, proteinase, or peptidase, any of a group of enzymes that break the long chainlike molecules of proteins into shorter fragments (peptides) and eventually into their components, amino acids.
Where can I get proteases?
So now that we know all that proteases can do, where can you get them from? As mentioned earlier, both plants and animals have proteases, and in some cases, incorporating plant enzymes is a great option. Two popular proteases that come from plant sources are papain from papayas and bromelain from pineapples. Both of these have been used for their ability to break down proteins for centuries, but as a meat tenderizer, not for health reasons.6 These are two of the most popular food sources, but there are others as well, such as ginger, asparagus, kiwifruit and kimchi. Another option is getting proteases from supplements for a variety of health support functions. For example, using protease in a plant-based digestive formula, will help with nutrient absorption while supporting digestive function; However, proteases are also used to help address excessive mucus due to allergies or temperature changes.
Why is protease used in plant based diet?
For example, using protease in a plant-based digestive formula, will help with nutrient absorption while supporting digestive function ; However, proteases are also used to help address excessive mucus due to allergies or temperature changes.
What are the main enzymes that make proteins?
The main way that our bodies do this is through enzymes, and in protein’s case, the primary enzymes that get the job done are proteases, also known as peptidases or proteinases.
Why is protein important?
Protein is generally hailed as one of the “building blocks” of the human body, being an essential component in many bodily structures as well as bodily processes. However, like every other type of nutrient, we need to be able to extract it from either our diet or another source. The main way that our bodies do this is ...
Does protease break down fat?
Compared to lipase and amylase, which break down fats and carbohydrates, respectively, the protease family has more extensive roles. Yes, protease helps break down protein in food into amino acids, which the body can then use for energy, but where proteases stand apart is the fact that they also play a number of other roles in essential processes, such as:
Does protease help with muscle soreness?
Muscle soreness: Athletes consider protein to be a major part of their health regimen, and protease may factor in as well. In one study, a protease enzyme blend reduced muscle tenderness and soreness post-workout over placebo.4.
Can proteases be taken with food?
There are two options to choose from, as proteases are available in digestive or systemic / therapeutic formulations. The former is taken with food and the latter, in most cases, is taken away from food. Please contact your Enzyme Science Representative for more information about the options that are available.
Where Does Protease Come From?
Your pancreas produces an inactive form of protease naturally. It's only after this inactive protease interacts with the enzyme trypsinogen that your body can use it for the above purposes. (2)
What is the role of proteases in the body?
Protease: Where It Fits In The World Of Enzymes. Proteases are one of three main types of digestive enzymes that your body needs. While we explored the other two in detail within our ' what are enzymes ' article, to summarize, the other two enzymes are lipase, which helps your body digest fat, and amylase, which helps your body process starches ...
What is the condition that reduces the ability of the pancreas to produce the digestive enzymes your body needs?
We'll explore pancreatic insufficiency in more detail shortly but in a nutshell, it's a condition that reduces your pancreas' ability to produce the digestive enzymes your body needs.
Why is protease so popular?
Supplements containing protease have become incredibly popular due to increased rates of digestive issues. Most often, as in the case of our protease supplement, you'll find it in formulas alongside other enzymes. This is because all enzymes work together for ensuring proper digestive function.
Why do enzymes work together?
This is because all enzymes work together for ensuring proper digestive function. Our modern diets can be messy sometimes and it's not always easy to get the amount of natural protease-containing foods that you need to combat the issue effectively.
What are the different types of proteases?
In this article, we'll be referring to proteases as a whole category. But here are the four main classes of proteases so you know what to look out for on ingredient labels and the like: 1 Serine 2 Cysterine 3 Aspartyl 4 Metalloprotease
What is lactase in milk?
Lactase is an ingredient that helps your body process milk and other dairy products. It's especially useful for people who suffer from lactose intolerance. If you're unfamiliar with kefir, it's a drink resembling a thin yogurt. To make it, you insert "kefir grains" (which resemble cauliflower) into milk.
Where is protein digested?
Protein is a vital nutrient for almost every part of your body. It’s digested in your mouth, stomach, and small intestine before it’s released into your bloodstream as individual amino acids.
How is protein absorbed?
These are small, finger-like structures that increase the absorptive surface area of your small intestine. This allows for maximum absorption of amino acids and other nutrients.
How can I absorb more protein?
The first step in increasing your protein absorption is choosing whole proteins that contain all nine essential amino acids. These include:
What enzyme breaks down carbohydrates and fats?
Protein digestion begins when you first start chewing. There are two enzymes in your saliva called amylase and lipase. They mostly break down carbohydrates and fats.
What is protein in the body?
What is protein? Protein is one of the most important substances in your body. Your muscles, hair, eyes, organs, and many hormones and enzymes are primarily made out of protein. It also helps to repair and maintain your body tissues. However, not all protein is created equal, and there are things you can do to help your body use it more efficiently.
How to maximize the nutrients you get from protein sources?
You can maximize the nutrients you get from protein sources by eating complete proteins and adopting certain habits, such as chewing thoroughly before swallowing.
Where is PR3 stored?
PR3 is stored in the azurophilic granules of neutrophils, but can also be found within the membrane of secretory vesicles, and also expressed at the plasma membrane. PR3 is physiologically inhibited by α1-antitrypsin [3].
What is the role of PR-3 in endothelial cells?
In the early stages of AAV, however, endothelial cells are known to recruit inflammatory cells and enhance their adhesion to sites of vascular injury . The subsequent release of PR-3 (from infiltrating leukocytes, at a minimum) and other neutrophil proteases may induce endothelial synthesis and secretion of IL-8, a potent neutrophil chemoattractant, thereby attracting additional neutrophils. PR-3 released by neutrophils can also enhance the adhesion of accumulating neutrophils and mononuclear cells to the endothelial surface via the induction of adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1). VCAM-1 is known to be expressed in situ within the renal lestions of patients with AAV. The soluble endothelial protein C receptor binds activated neutrophils via interactions with PR-3, providing a link between neutrophil priming, vascular inflammation, and the coagulation cascade. This may explain, in part, the increased risk of venous thrombotic events observed in WG. 16 Organ-specific anti-endothelial antibodies have also been reported, albeit the precise antigens and role in disease development are unclear.
What is PR3 in biology?
PR3 is a multifunctional protein. Several data support a significant role of PR3 in the regulation of myeloid differentiation. Upregulation of PR3 causes growth of pregenitor cells, but is subsequently downregulated after the promyelocytic stage and growth arrest and further differentiation along the granulocytic lineage is induced. Most of the biologic functions ascribed to PR3 are dependent on its proteolytic activity. The physiologic function of PR3 is degradation of extracellular proteins at sites of inflammation including proteolysis of elastin, hemoglobin, fibronectin, laminin, and collagen type IV. PR3 has been implicated in the modulation of inflammatory mediators: it is involved in the cleavage and activation and/or inactivation of C1 inhibitor, membrane-bound tumor necrosis factor (TNF)-α, interleukin (IL)-2 receptor, IL-6, transforming growth factor (TGF)-β, and protease-activated receptor (PAR)-2 [4]. Moreover, PR3 has nonproteolytic antimicrobial activities against Candida albicans and Escherichia coli. PR3 may modulate the activity of platelets, endothelial cells, and dendritic cells. Among PR3 interaction partners (CD16, CD11b/CD18), CD177, a glycosylphosphatidylinositol (GPI)-anchored membrane protein is a potential receptor for soluble PR3. In addition, PR3 can be externalized at the plasma membrane at a very early stage of neutrophil apoptosis, in the absence of degranulation. In these conditions, PR3 is associated with specific partners including phospholipid scramblase-1 and calreticulin. Interestingly, apoptosis-induced PR3 membrane expression significantly impaired macrophage phagocytosis. This new role of PR3 acting as a “don’t eat me signal” that delays neutrophil clearance might potentiate inflammation and autoimmunity [3].
What is PR3 in C-ANCA?
PR3 (EC 3.4.21.76), the main target of C-ANCA, is a cationic protein (isoelectric point pI 9.4) consisting of 228 amino acid residues and belonging to the trypsin family of serine proteases. PR3 was shown to be identical to other molecules described as AGP7 (azurophil granule protein, neutrophil protease p29, and myeloblastin). The gene for PR3 is localized on chromosome 19p13.3, spans 6570 base pairs, and consists of five exons and four introns. During translation, PR3 is synthesized as a prepro-enzmye and subsequently processed in four steps into the mature form consisting of 222 amino acids. PR3 is stored in the azurophilic granules of neutrophils, but can also be found within the membrane of secretory vesicles, and also expressed at the plasma membrane. PR3 is physiologically inhibited by α1-antitrypsin [3].
How many amino acids are in proteinase 3?
Proteinase 3, a 29–30 kDa serine-protease consisting of 228 aminoacids, is a weak cationic enzyme, physiologically inhibited by α1-antitrypsin [3 ]. It has a structure with considerable homology to neutrophil elastase. By aminoacids sequencing, the protein was found to be identical to previously described p29, azurophil granule protein (AGP7), and myeloblastin [ 3 ].
What is the first protocol for purification of PR3?
The first is based on dye ligand (Orange A) affinity binding of PR3 and subsequent purification of the antigen through cation exchange chromatography.
Where is PR3 translocated?
Recently, PR3 was detected in human endothelial cells ( Mayet et al., 1993 ). PR3 is translocated to the endothelial cell-membrane under the influence of cytokines, becoming accessible to cANCA. We investigated the effect of 5/7Id on the binding of cANCA to cytokine-treated endothelial cells.
Which enzyme is located in the wall of the small intestine?
elastase. pancreatic enzyme that digests protein. enterokinase. enzyme located in the wall of the small intestine that activates trypsin. inactive proenzymes. forms in which proteases are stored and released to prevent the inappropriate digestion of the native proteins of the stomach, pancreas, and small intestine.
Where does the urea cycle occur?
It occurs primarily in the liver and, to a lesser extent, in the kidney. Prior to the urea cycle, ammonium ions are produced from the breakdown of amino acids.
Why are pancreatic enzymes released?
In order to avoid breaking down the proteins that make up the pancreas and small intestine, pancreatic enzymes are released as inactive proenzymes that are only activated in the small intestine. In the pancreas, vesicles store trypsin and chymotrypsin as trypsinogen and chymotrypsinogen.
What is the name of the enzyme that converts pyruvate into phenylketonuria?
Metabolism: Pyruvate Dehydrogenase Complex Deficiency and Phenylketonuria. Pyruvate dehydrogenase complex deficiency (PDCD) and phenylketonuria (PKU) are genetic disorders. Pyruvate dehydrogenase is the enzyme that converts pyruvate into acetyl CoA, the molecule necessary to begin the Krebs cycle to produce ATP.
What enzymes are released by secretin?
Secretin also stimulates the pancreas to release sodium bicarbonate. The pancreas releases most of the digestive enzymes, including the proteases trypsin, chymotrypsin, and elastase, which aid protein digestion.
How are peptides transported?
These smaller peptides are catabolized into their constituent amino acids, which are transported across the apical surface of the intestinal mucosa in a process that is mediated by sodium-amino acid transporters. These transporters bind sodium and then bind the amino acid to transport it across the membrane.
What is the pH of proteins?
The latter produces an environmental pH of 1.5–3.5 that denatures proteins within food.
