
List of all the metals on the Periodic table
| Atomic number | Symbol | Name of element |
| 3 | Li | Lithium |
| 4 | Be | Beryllium |
| 11 | Na | Sodium |
| 12 | Mg | Magnesium |
Where can the most abundant element be found?
- The Earth's crust makes up 1% of the planet's volume. Many elements make up the crust, with some being more abundant than others.
- Oxygen, silicon, iron and aluminum are the most abundant elements in the Earth's crust, accounting for 88.1% of its mass.
- No-one has ever journeyed to the center of the Earth. ...
Where are elements and compounds usually found?
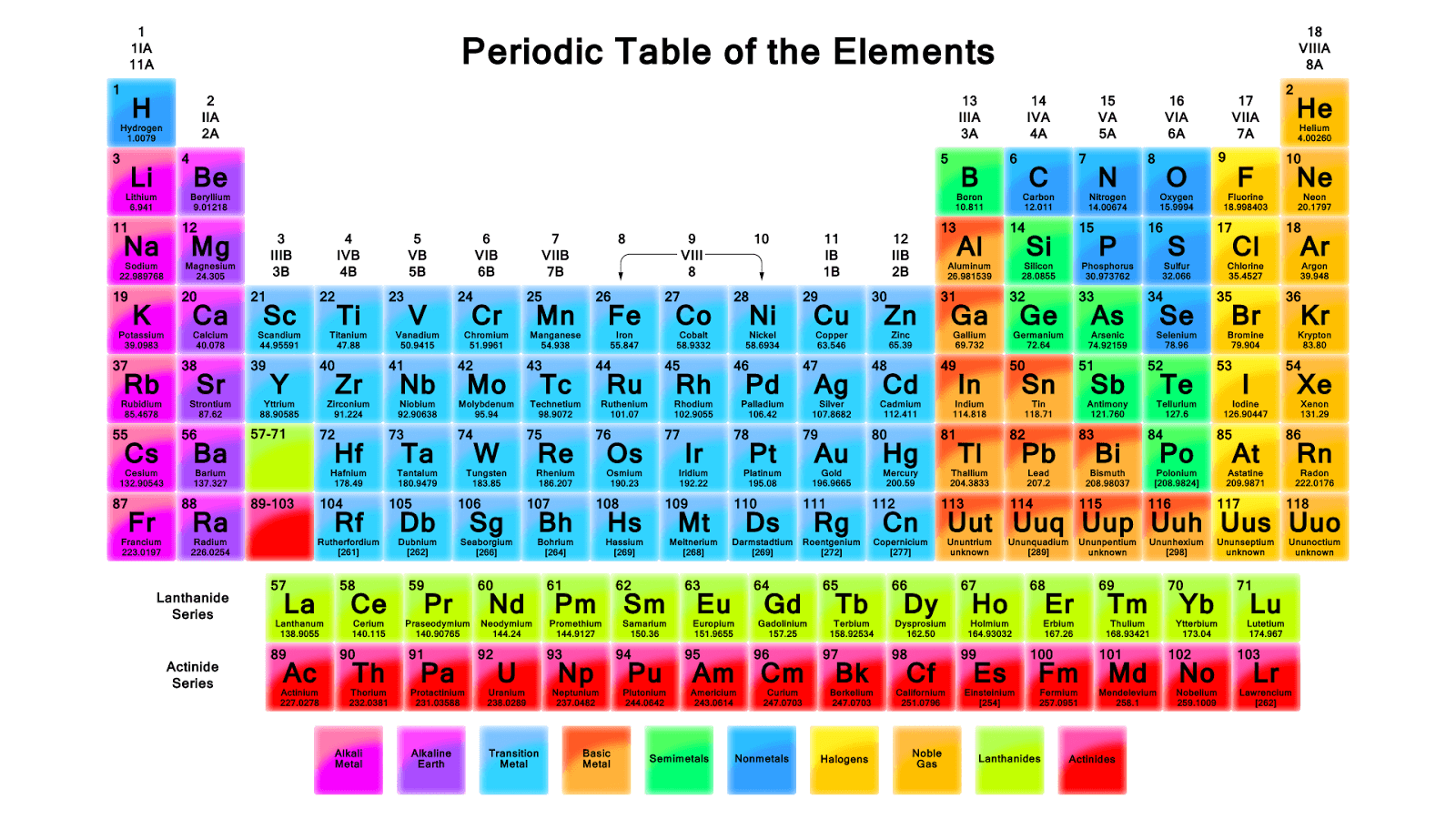
Most elements are metals, which are found on the left and toward the bottom of the periodic table. A handful of nonmetals are clustered in the upper right corner of the periodic table. The semimetals can be found along the dividing line between the metals and the nonmetals. Atoms.
Where are the rare earth elements located?
Where are rare earth metals found? Estimates believe that rare earth deposits are mostly found in Asia, although stocks varied dramatically by country.
Which elements are found in the lithosphere?
- Lithosphere: The lithosphere is the solid part of Earth, including the crust. It contains rocks and minerals. ...
- Biosphere: The biosphere contains Earth’s living matter. All plants and animals are part of this sphere. ...
- Hydrosphere: All of the water on Earth is part of the hydrosphere. ...
- Atmosphere: The atmosphere includes the air that surrounds Earth. ...
What is an element and where is it found?
A chemical made up of only one kind of atom is called an element. There are 118 different elements, although only 98 of them are found naturally on Earth. Some elements, such as gold, are found in their pure form on Earth. Others, like iron, have to be separated from other substances.
How are the elements found on Earth?
The elements found on earth originate from stars or the big bang. Most of the hydrogen and helium on earth originated just after the big bang. The heavier elements like silicon and oxygen were mainly made on stars. Some of the more common elements such as iron were made by a process known as nuclear fusion.
Where did Earth get all its elements?
Hydrogen and helium, the lightest elements, were formed about 14 billion years ago in the first few minutes following the big bang—the beginning of the universe. Trace amounts of lithium were also formed then. Every other chemical element was produced by stars and cosmic events as the universe evolved.
Are all elements found naturally on Earth?
Elements 1 through 92 (except for elements 43 and 61) occur naturally on Earth, although some are only present in extremely small quantities. The elements following uranium on the periodic table are only produced artificially, and are known as the transuranium or transuranic elements.
Science Lesson: Earth, Water, Air, and Fire
The ancient Greeks believed that there were four elements that everything was made up of: earth, water, air, and fire. This theory was suggested ar...
Science Lesson: The Four Elements in Everyday Life
The earth is full of a wide variety of rocks and minerals which provides the soil to grow vegetation and support life. The two most common elements...
Science Projects: Exploring The Four Elements
In order to put out a fire, one of three things must be removed from it: heat, fuel, or oxygen. Knowing this, firefighters don’t always use water t...
What are the two elements that were discovered in nature?
Neptunium and plutonium were also discovered in nature, in uranium-rich rock. These two elements resulted from neutron capture by uranium: The key takeaway here is that bombarding an element with neutrons can produce new elements because neutrons can turn into protons via a process called neutron beta decay.
How are new elements made?
A new element is made by adding a proton (or more than one) or neutron to a pre-existing element. This can be done by smashing protons or neutrons into atoms or by colliding atoms with each other.
Why are nuclei not enough to form an element?
Nuclear reactors and particle accelerators can bombard targets with neutrons, protons, or atomic nuclei. To form elements with atomic numbers greater than 118 , it's not enough to add a proton or neutron to a pre-existing element. The reason is that the superheavy nuclei that far into the periodic table simply aren't available in any quantity and don't last long enough to be used in element synthesis. So, researchers seek to combine lighter nuclei that have protons that add up to the desired atomic number or they seek to make nuclei that decay into a new element. Unfortunately, because of the short half life and the small number of atoms, it's very hard to detect a new element, much less verify the result. The most likely candidates for new elements will be atomic number 120 and 126 because they are believed to have isotopes that might last long enough to be detected.
Why is it so hard to detect new elements?
Unfortunately, because of the short half life and the small number of atoms, it's very hard to detect a new element, much less verify the result. The most likely candidates for new elements will be atomic number 120 and 126 because they are believed to have isotopes that might last long enough to be detected.
What are the processes that make new elements?
The Processes That Make New Elements. The elements found on Earth today were born in stars via nucleosynthesis or else they formed as decay products. All of the elements from 1 (hydrogen) to 92 (uranium) occur in nature, although elements 43, 61, 85, and 87 result from radioactive decay of thorium and uranium.
Who created the periodic table?
Dmitri Mendeleev is credited with making the first periodic table that resembles the modern periodic table. His table ordered the elements by increasing atomic weight (we use atomic number today ). He could see recurring trends, or periodicity, in the properties of the elements. His table could be used to predict the existence ...
Why are heavier elements found in stars?
Some heavier elements are likely made within stars, but because they have such short half-lives, they haven't survived to be found on Earth today. At this point, the problem is less about making new elements than detecting them. The atoms that are produced often decay too quickly to be found.
Rare Earth Elements: Where in the World Are They?
Rare earth elements are a group of metals that are critical ingredients for a greener economy, and the location of the reserves for mining are increasingly important and valuable.
Visualizing Nuclear Power Production by Country
Nuclear power accounted for 10% of global electricity generated in 2020. Here’s a look at the largest nuclear power producers.
What element is used in batteries?
3. Lanthanum (La): This rare element is used in the nickel metal hydride (NiMH) batteries found in some smartphones, laptops, and electric cars. It was also discovered by Mosander. 4. Neodymium (Nd): One of its non-electronic uses is to colour glass.
What element is used in nuclear reactors?
1. Europium (Eu): Discovered by Frenchman Eugene-Anatole Demarcay in 1896, this element is used in nuclear reactors as well as low-energy light bulbs and TV.
What is the name of the metal that is found in LCD screens?
2. Terbium (Tb): Swedish chemist Carl Mosander discovered this soft, malleable and ductile metal in 1843, which is now found in the make-up of LCD screens and memory devices including the ubiquitous USB drive. Frustrated Things GIF - Find & Share on GIPHY. via GIPHY. 3.
What are the most common elements in the periodic table?
The most common of these elements are sodium and potassium. Rubidium, lithium, and cesium are more rare, making up, in order, 0.03, 0.007, and 0.0007 percent of the Earth’s crust.
Where is lithium found?
Discovered in 1817, lithium is the lightest of all metals. It does not occur freely in nature and is found (combined) in all igneous rocks, mineral springs, and the minerals lepidolite, spodumene, petalite, and amblygonite.
How many isotopes does oxygen have?
Oxygen is a component of hundreds of thousands of organic compounds and readily combines with most elements. Oxygen has nine isotopes. Its allotrope ozone (O 3) is formed when oxygen is subjected to an electrical discharge or ultraviolet light.
What are the rarest elements in the Earth's crust?
Rubidium, lithium, and cesium are more rare, making up, in order, 0.03, 0.007, and 0.0007 percent of the Earth’s crust. These elements are very reactive, and usually occur in nature already combined with other elements.
How much of the atmosphere is oxygen?
Oxygen gas forms 21% of the atmosphere by volume and the element and its compounds make up nearly half the weight of the earth's crust. Two thirds of the human body and nine tenths of water are oxygen.
What can you use to sort the periodic table?
You may also use the color-coded periodic table chart with names, symbols, and atomic weights to find specific information you need for your work. Easy-to-use filters allow you to sort by metals, nonmetals, physical states, group, period, and more.
How are noble gases extracted from the air?
Except for helium and radon, noble gases can be extracted from the air using liquefaction and fractional distillation. Helium is obtained from natural gas wells and radon is a product of radioactive decay.
What is the most common element in the human body?
Most of the human body is made up of water, H 2 O, with bone cells being comprised of 31% water and the lungs 83%. 1 Therefore, it isn't surprising that most of a human body's mass is oxygen. Carbon, the basic unit for organic molecules, comes in second. 96.2% of the mass of the human body is made up of just four elements: oxygen, carbon, ...
What are the elements that make up the human body?
96.2% of the mass of the human body is made up of just four elements: oxygen, carbon, hydrogen, and nitrogen. Oxygen (O) - 65% - Oxygen together with hydrogen form water, which is the primary solvent found in the body and is used to regulate temperature and osmotic pressure.
What are the elements that are essential for life?
Trace elements considered essential in humans include zinc, selenium, nickel, chromium, manganese, cobalt, and lead. Not all of the elements found within the body are essential for life. Some are considered contaminants that appear to do no harm but serve no known function. Examples include cesium and titanium.
Where is hydrogen found?
Hydrogen (H) - 9.5% - Hydrogen is found in water and in all organic molecules.
What is magnesium 0.1%?
Magnesium (Mg) - 0.1% - Magnesium is involved in over 300 metabolic reactions. It's used to build the structure of muscles and bones and is an important cofactor in enzymatic reactions.
How many elements will be discovered in 2021?
The discovery of the 118 chemical elements known to exist as of 2021 is presented in chronological order. The elements are listed generally in the order in which each was first defined as the pure element, as the exact date of discovery of most elements cannot be accurately determined. There are plans to synthesize more elements, ...
What was the last element discovered in nature?
Francium was the last element to be discovered in nature, rather than synthesized in the lab, although four of the "synthetic" elements that were discovered later (plutonium, neptunium, astatine, and promethium) were eventually found in trace amounts in nature as well. 93.
What is the oldest iron?
The oldest known iron objects used by humans are some beads of meteoric iron, made in Egypt in about 4000 BC. The discovery of smelting around 3000 BC led to the start of the Iron Age around 1200 BC and the prominent use of iron for tools and weapons. 6. Carbon.
Where are copper beads found?
Copper beads dating from 6000 BC have been found in Çatal Höyük, Anatolia and the archaeological site of Belovode on the Rudnik mountain in Serbia contains the world's oldest securely dated evidence of copper smelting from 5000 BC. 82. Lead.
Who discovered the isotope of radium?
In 1900, Friedrich Ernst Dorn discovered a longer-lived isotope of the same gas from the radioactive decay of radium. Since "radon" was first used to specifically designate Dorn's isotope before it became the name for the element, he is often mistakenly given credit for the latter instead of the former. 89.
How many elements did Lavoisier write?
Lavoisier writes the first modern list of chemical elements – containing 33 elements including light, heat, unextracted "radicals" and some oxides. He also redefines the term "element". Until then, no metals except mercury were considered elements.
What are the elements of medieval alchemy?
The first four are always found. The fifth, aether, is important in some traditions. Sulfur, mercury, and salt are classical elements . air.
What are the elements of Akasha?
Although the names are different, the first four elements roughly translate as being air, fire, water, and earth. Vayu (wind or air)
