What are the 3 states of water?
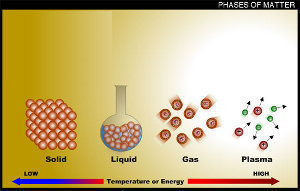
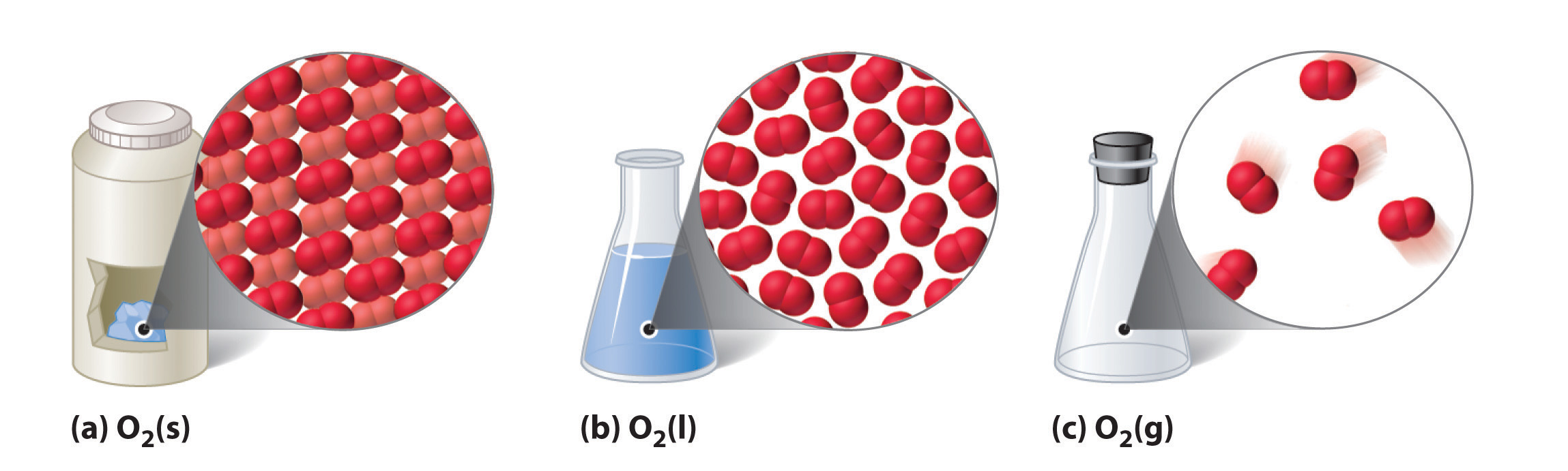
States of Water. Water can exist in 3 different physical states – solid, liquid, and gaseous. In the solid state, water exists in the form of ice or snow. In the gaseous state, water exists as water vapour or steam. Finally, the liquid state of water (which is the state in which it is consumed by living organisms) is simply referred to as ‘water’.
What is the state of water in the solid state?
In the solid state, water exists in the form of ice or snow. In the gaseous state, water exists as water vapour or steam. Finally, the liquid state of water (which is the state in which it is consumed by living organisms) is simply referred to as ‘water’.
What is the state of water in everyday life?
Water is the only substance where all three states can be readily observed in everyday life: solid water as ice, liquid water in a water fountain, and gaseous water as steam. Why is water in liquid form? At room temperature (anywhere from zero degree centigrade to 100 degrees centigrade), water is found in a liquid state.
What is the state of matter of water vapour?
Water vapour (sometimes referred to as steam, especially when it holds temperatures above 100 o C) is the gaseous state of water. Liquid water can enter the gaseous phase via two different processes – evaporation and boiling.
What are the states of water?
States of Water. Water can exist in 3 different physical states – solid, liquid, and gaseous. In the solid state, water exists in the form of ice or snow. In the gaseous state, water exists as water vapour or steam. Finally, the liquid state of water (which is the state in which it is consumed by living organisms) is simply referred to as ‘water’.
How does liquid water enter the gaseous phase?
Liquid water can enter the gaseous phase via two different processes – evaporation and boiling. The former involves the escape of water molecules from the surface into the atmosphere whereas the latter involves the transfer of thermal energy to the water molecules at the bulk in order to facilitate a phase transition.
Why is water important to life?
This is because liquid water functions as a universal solvent and provides the environment for many biochemical reactions.
What temperature does water melt?
When solid water is heated to temperatures above 0 degrees celsius, it melts and reverts to its liquid state. This temperature point on the celsius scale corresponds to a value of 32 degrees on the Farenheit scale and 273.15 on the Kelvin scale. The solid form of water has numerous cooling-based applications. For example, ice is often added to cold-drinks and other beverages to reduce the temperature of the beverage.
What is liquid water used for?
Furthermore, liquid water is also used for washing clothes and for other cleaning activities (such as bathing). Liquid water is also used industrially as a solvent for the manufacture of several commercially important goods.
Why does ice float on water?
Due to the fixed intermolecular distances in the solid state of water, the density of ice is lower than the density of liquid water. This is the reason why ice floats on water. Therefore, when the weights of equal volumes of ice and water are compared, the weight of the water will almost always be higher.
What is the freezing point of water?
The Solid State of Water – Ice. The freezing point of water under standard conditions for temperature and pressure (STP) corresponds to 0 o C. When liquid water is cooled to this temperature, the substance freezes and enters the solid state.
What are the three states of water?
States of Water: Gas, Liquid and Solid . Because water is extremely versatile, it can change states into the following three forms: Let’s take a look at the conditions water needs to transform states.
Why does water absorb energy?
Because the water molecules absorb energy from heat, these energetic molecules stay farther apart from each other. It takes a tremendous amount of energy to turn a liquid into a gas. For example, it requires 540 calories per gram for evaporation to occur.
How does evaporation convert liquid water to vapor?
If you add enough energy from the sun, water evaporates into a gas from a liquid. In other words, it breaks the covalent bond of water.
How does condensation go from water vapor to liquid?
Instead of gaining heat, water molecules lose energy from a loss of heat during condensation. In turn, they bond together from a gas into a liquid state.
What is the beginning of the hydrological cycle?
Overall, the start of the hydrological cycle begins with evaporation at the surface of the ocean. Next, water condenses and precipitates back into the ocean, streams, glaciers, or groundwater. Eventually, it makes its way back to the ocean. And that’s the whole cycle.
What are the last two phases of a gas?
Finally, the last two phases are deposition and sublimation which are complete opposites of each other. Sublimation is when a solid changes state to a gas, without going into the intermediate liquid phase. Whereas deposition is when a gas transforms into a solid-state.
What is the process of forming tiny water droplets?
In turn, they bond together from a gas into a liquid state. Condensation is the process of forming tiny water droplets. They surrounding a particle of dust or salt. Eventually, they become too heavy and fall as precipitation. For example, liquid water drips down a chilled glass of lemonade on a hot day because of condensation.