How to increase acetylcholine levels naturally?
- Meditate
- Practice deep breathing
- Do Yoga
- Listen to calming music
How do you produce more acetylcholine?
To increase levels of acetylcholine, we use the following approach:
- Create more acetyl-CoA molecules to provide acetyl groups to ChAT for acetylcholine production.
- Provide more choline in choline producing ( cholinergic) neurons for use by ChAT.
- Increase or upregulate the activity of ChAT.
- Decrease the activity of AchE.
Is too much acetylcholine bad?
Too high acetylcholine primarily operates by inhibiting other neurotransmitters. The symptoms of too high acetylcholine may be similar to the symptoms of too low serotonin, as they have a close balancing relationship. Once we have identified potential neurotransmitter imbalances, it is time to treat them.
What are the 7 types of neurotransmitters?
TYPES OF NEUROTRANSMITTERS BOTH Acetylcholine Nor epinephrine EXCITATORY Glutamate Aspartate Nitric oxide INHIBITORY Glycine GABA Serotonin Dopamine 7. ACETYLCHOLINE (ACh) Acetylcholine was the first neurotransmitter to be discovered. Isolated in 1921 by a German biologist named Otto Loewi. Uses choline as a precursor - cholinergic ...

Where does acetylcholine synthesized?
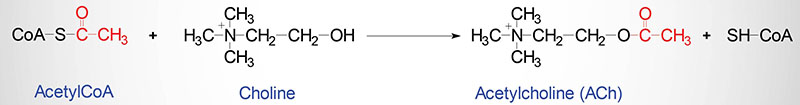
nerve terminalsAcetylcholine is synthesized in nerve terminals from acetyl coenzyme A (acetyl CoA, which is synthesized from glucose) and choline, in a reaction catalyzed by choline acetyltransferase (CAT) (Figure 6.8).
Where are acetylcholine stored?
nerve terminalsAcetylcholine is stored in nerve terminals and released by nerve depolarization. Released acetylcholine binds to postsynaptic muscarinic and/or nicotinic receptors.
What is acetylcholine storage?
vesiclesAcetylcholine is stored until the neurotransmitter needs to be released. After its synthesis, acetylcholine (ACh) is packaged into vesicles. ACh is transported from the cytoplasm into individual vesicles by means of a carrier protein on the vesicle membrane called the vesicular ACh transporter (VAChT).
How is acetylcholine stored in the neuron?
ACh is transported into storage vesicles following its synthesis by ChAT in the nerve ending [16]. The vesicular ACh transporter (VAChT) has been cloned and expressed. Its sequence places it in the 12-membrane-spanning family characteristic of other biogenic amine transporters found in adrenergic nerve endings [17,18].
Is acetylcholine stored in synaptic vesicles?
An oblique fracture of the nerve terminal reveals a number of larg er organelles in the nerve cell called synaptic vesicles. The vesicles store acetylcholine, and it has long been thought that acetylcholine is released when the vesicles fuse with the cell membrane and re lease their contents into the synaptic cleft.
How is acetylcholine made stored and released?
Acetylcholine is synthesized in cholinergic neurons and is the principal regulator of GI motility and pancreatic secretion. Acetylcholine is stored in nerve terminals and released by nerve depolarization. Released acetylcholine binds to postsynaptic muscarinic and/or nicotinic receptors.
Where is acetylcholine synthesized quizlet?
ACh is synthesized from choline (obtained through fats in our diet) and acetyl coenzyme A (generated through metabolism of sugars and fats). Enzyme that synthesizes Ach from acetyl CoA and choline . Where is ChAT located? ChAT is only located in the cytoplasm of the neurons that use ACh.
Where are neurotransmitters stored?
Neurotransmitters are located in a part of the neuron called the axon terminal. They're stored within thin-walled sacs called synaptic vesicles. Each vesicle can contain thousands of neurotransmitter molecules.
Why are neurotransmitters stored in vesicles?
In a neuron, synaptic vesicles (or neurotransmitter vesicles) store various neurotransmitters that are released at the synapse. The release is regulated by a voltage-dependent calcium channel. Vesicles are essential for propagating nerve impulses between neurons and are constantly recreated by the cell.
What nerve releases acetylcholine?
Preganglionic sympathetic and parasympathetic fibers both use acetylcholine as neurotransmitter and the postganglionic cells have nicotinic cholinergic receptors. The postganglionic parasympathetic nerves also release acetylcholine, and the postsynaptic target cells have muscarinic cholinergic receptors.
How is acetylcholine released from the axon terminal?
Acetylcholine is stored in the terminal in small sacs, or vesicles. When an electrical impulse originating in the cell body travels down the axon to the terminal, it triggers the release of acetylcholine from the vesicles into the synaptic space (also called the synapse2) (Figure 5).
How is acetylcholine removed from the synapse?
Acetylcholine is removed from the synaptic cleft by an specialized enzyme located in the synaptic cleft called acetylcholinesterase (AChE). AChE is present in all cholinergic synapses to quickly inactivate this excitatory neurotransmitter. The AChE breaks acetylcholine into two parts: acetate and choline.
Which division of the nervous system consists of the brain and spinal cord quizlet?
Which division of the nervous system consists of the brain, spinal cord, and optic nerves? Central nervous system.
Where are neurotransmitters stored?
Neurotransmitters are located in a part of the neuron called the axon terminal. They're stored within thin-walled sacs called synaptic vesicles. Each vesicle can contain thousands of neurotransmitter molecules.
Where are receptors for acetylcholine located in a nerve cell?
Acetylcholine receptors (also called cholinergic receptors) appear in clusters on muscle-cell membranes opposite the active zones of presynaptic terminals. Their density at these receptor regions is between 7,000 and 30,000 sites per square micrometre (micron; millionth of a metre).
Where are receptors for acetylcholine located quizlet?
-Receptors for acetylcholine are located on the motor end plate -- the portion of the muscle fiber's sarcolemma that faces the neuron's synaptic terminal. Binding of acetylcholine to acetylcholine receptors increases the sodium permeability of the motor end plate.
What is the acetylcholine transporter?
A specific low-affinity acetylcholine transporter is responsible for uptake of the transmitter from the cytoplasm into vesicles. The genes for choline acetyltransferase and the vesicular acetylcholine transporter are organized in a single gene locus, and transcription of the two genes is typically co-regulated. (±)-Vesamicol is a selective inhibitor of this transporter, with L- (–)-vesamicol being more potent than D- (+)-vesamicol. Once packaged in vesicles, acetylcholine is subject to stimulus-induced release by exocytosis. Several powerful toxins impact on acetylcholine release, notably botulinum toxin which inhibits its release.
What toxins inhibit acetylcholine release?
Several powerful toxins impact on acetylcholine release, notably botulinum toxin which inhibits its release. Neuronal acetylcholinesterase very rapidly inactivates the majority of acetylcholine released in brain, although butyrylcholinesterase contained in glial cells may hydrolyze a small proportion of acetylcholine in the synapse.
How is enzyme activity regulated?
Enzyme activity is also regulated by product inhibition; by binding at an allosteric site on choline acetyltransferase, acetylcholine inhibits its activity. In addition, mass action and neuronal activity influence the rate of acetylcholine formation. Short-term regulation of enzyme activity is partly achieved by phosphorylation induced by protein ...
Why are cholinesterases withdrawn from clinical use?
Some second generation cholinesterases have been withdrawn from clinical use because of unacceptable side effects (e.g, tacrine, metrifonate ). Irreversible acetylcholinesterase inhibitors are used as insecticides and chemical warfare agents. Choline, which is liberated from acetylcholine by acetylcholinesterase, ...
Which cell is responsible for transporting choline into the brain?
There is a carrier system in capillary endothelial cells that is responsible for transport of choline, in its free and phospholipid forms, into the brain. Hydrolysis of choline-containing phospholipids may also liberate choline that is used in acetylcholine synthesis. As choline acetyltransferase is not saturated by concentrations ...
Where is acetylcholine synthesized?
In the nervous system, this enzyme is thought to exist primarily in the nerve terminal cytoplasm. Coenzyme A is synthesized in mitochondria and accesses choline acetyltransferase following transport across the mitochondrial membrane into the cytoplasm. In addition to its synthesis in the liver, choline employed in acetylcholine production is derived from dietary sources. There is a carrier system in capillary endothelial cells that is responsible for transport of choline, in its free and phospholipid forms, into the brain. Hydrolysis of choline-containing phospholipids may also liberate choline that is used in acetylcholine synthesis. As choline acetyltransferase is not saturated by concentrations of acetyl coenzyme A and choline that are estimated to be present in the nerve terminal, it appears that the rate of acetylcholine synthesis is dependent on precursor availability. Enzyme activity is also regulated by product inhibition; by binding at an allosteric site on choline acetyltransferase, acetylcholine inhibits its activity. In addition, mass action and neuronal activity influence the rate of acetylcholine formation. Short-term regulation of enzyme activity is partly achieved by phosphorylation induced by protein kinases. There are no very specific and potent inhibitors of the enzyme and it should be noted that pharmacological blockade of this step (e.g. with naphthylvinylpyridine) in the life-cycle of acetylcholine produces a less profound effect on the transmitter than does inhibition of choline transport.
Where is coenzyme A synthesized?
Coenzyme A is synthesized in mitochondria and accesses choline acetyltransferase following transport across the mitochondrial membrane into the cytoplasm. In addition to its synthesis in the liver, choline employed in acetylcholine production is derived from dietary sources. There is a carrier system in capillary endothelial cells ...
Which receptors bind to Ach?
To elicit a response, Ach binds with the available cholinergic receptors on the postsynaptic membrane. There are mainly two types of cholinergic receptors-nico tinic and muscarinic. These are again having subtypes like nicotinic N m (on skeletal muscle cell) and N n (neuronal) and muscarinic M 1, M 2, M 3, M 4 and M 5 receptors.
Where is ach synthesized?
Synthesis, Storage and Release of Ach: Ach is synthesized in the cholinergic nerve endings. After a reaction among acetate, coenzyme A and ATP, acetyl CoA is formed within the mitochondria and released into the cytoplasm. Choline enters into the axoplasm by active transport through the axonal membrane. ADVERTISEMENTS:
When an action potential arrives at the motor or cholinergic nerve terminal, depolarization of?
When an action potential arrives at the motor or cholinergic nerve terminal, depolarization of the area opens the voltage-gated Ca 2+ channels on the axonal membrane, through which Ca 2+ enters into the axoplasm and helps in fusion of vesicles with axonal membrane, resulting in extrution of a larger quantity of Ach.
What are the functions of muscarinic receptors?
The basic functions of muscarinic receptors are mediated by the membrane bound G-proteins. The M 1, M 3 and M 5 receptors activate a G-protein (G q/11) which in term stimulates membrane bound phospholipase C (enzyme) activity resulting in hydrolysis of membranous phospholipids to form inositol triphosphate (IP 3) and diacylglycerol (DAG).
What receptors inhibit adenylyl cyclase?
M 2 and M 4 receptors interact with distinct group of G-proteins (Gi and Go) to inhibit the adenylyl cyclase, to activate receptor operated K + channels (in heart), and to suppress the activity of voltage-gated Ca 2+ channels in some cells.
What receptors stimulate nitric oxide release?
M 3 receptors , located on endothelial cells of blood vessels, stimulate nitric oxide (NO) (previously recognised as Endothelium dependent relaxing factor (EDRF)) release from the cells. NO then diffuses to the vascular smooth muscle to cause relaxation by stimulating cytosolic guanylyl cyclase enzyme.
What causes excessive Ach release?
The release of Ach can be inhibited by excess Mg 2+, botulinus toxin, or procaine. Black widow spider venom’ causes release of excessive amounts of Ach followed by blockade of release.
What is the mechanism of acetylcholine?
This paper has reviewed the main mechanisms of ACh synthesis, storage , and release. Presynaptic choline transport supports ACh production and release, and cholinergic terminals express a unique transporter critical for neurotransmitter release. Neurons cannot synthesize choline, which is ultimately derived from the diet and is delivered through the blood stream. ACh released from cholinergic synapses is hydrolyzed by acetylcholinesterase into choline and acetyl coenzyme A and almost 50% of choline derived from ACh hydrolysis is recovered by a high-affinity choline transporter. Parallel with the development of cholinergic hypothesis of geriatric memory dysfunction, cholinergic precursor loading strategy was tried for treating cognitive impairment occurring in Alzheimer's disease. Controlled clinical studies denied clinical usefulness of choline and lecithin (phosphatidylcholine), whereas for other phospholipids involved in choline biosynthetic pathways such as cytidine 5'-diphosphocholine (CDP-choline) or alpha-glyceryl-phosphorylcholine (choline alphoscerate) a modest improvement of cognitive dysfunction in adult-onset dementia disorders is documented. These inconsistencies have probably a metabolic explanation. Free choline administration increases brain choline availability but it does not increase ACh synthesis/or release. Cholinergic precursors to serve for ACh biosynthesis should be incorporate and stored into phospholipids in brain. It is probable that appropriate ACh precursors and other correlated molecules (natural or synthesized) could represent a tool for developing therapeutic strategies by revisiting and updating treatments/supplementations coming out from this therapeutic stalemate.
What is acetylcholine synthesis?
Pathways of acetylcholine synthesis, transport and release as targets for treatment of adult-onset cognitive dysfunction. Acetylcholine (ACh) is a neurotransmitter widely diffused in central, peripheral, autonomic and enteric nervous system.
Does free choline increase ACh?
These inconsistencies have probably a metabolic explanation. Free choline administration increases brain choline availability but it does not increase ACh synthesis/or release. Cholinergic precursors to serve for ACh biosynthesis should be incorporate and stored into phospholipids in brain.
How is epinephrine synthesized?
Figure 9.8. Epinephrine is synthesized from norepinephrine by phenylethanolamine-N-methyltransferase in the cytoplas m. Epinephrine is then packaged into vesicles by vesicular monoamine transporter. ‘Epinephrine Synthesis’ by Casey Henley is licensed under a Creative Commons Attribution Non-Commercial Share-Alike (CC BY-NC-SA) 4.0 International License.
How is glutamine converted into glutamate?
In the presynaptic terminal, glutamine is converted into glutamate via the enzyme glutaminase, which is the rate-limiting step in the synthesis pathway. Glutamate is packaged into vesicles for storage via the vesicular glutamate transporter. Figure 9.3.
How is glutamate synthesized?
Figure 9.3. Glutamate is synthesized from glutamine by glutaminase, the rate-limiting step in the pathway. Glutamate is then packaged into vesicles by vesicular glutamate transporter. ‘Glutamate Synthesis’ by Casey Henley is licensed under a Creative Commons Attribution Non-Commercial Share-Alike (CC BY-NC-SA) 4.0 International License.
What is glutamate used for?
GABA. Glutamate is then used to synthesize GABA, another amino acid transmitter and the primary inhibitory neurotransmitter in the brain. In the presynaptic terminal, glutamate is converted into GABA via the enzyme glutamic acid decarboxylase, which like the other synthesis pathways is the rate-limiting step.
What are the two main groups of small molecule transmitters?
The small molecule transmitters can be divided into two main groups: amino acid neurotransmitters and biogenic amines , also called monoamines. In addition to acting as neurotransmitters, the amino acids glutamate and glycine are used to synthesize proteins in all cell types throughout the body.
What are the two criteria for a neurotransmitter?
Resources. A few criteria must be met for a molecule to be called a neurotransmitter. First, the transmitter must be synthesized within in the presynaptic neuron. Second, the transmitter must be released by the presynaptic neuron in response to stimulation.
Where are neurotransmitters synthesized?
Most small molecule neurotransmitters are synthesized by enzymes that are located in the cytoplasm (the exception is norepinephrine, see below). This means that small molecule neurotransmitters can be synthesized and packaged for storage in the presynaptic terminal using enzymes present in the terminal.
Abstract
Acetylcholine is one of the major modulators of brain functions and it is the main neurotransmitter at the peripheral nervous system. Modulation of acetylcholine release is crucial for nervous system function. Moreover, dysfunction of cholinergic transmission has been linked to a number of pathological conditions.
1. Introduction
Most classical neurotransmitters are synthesized in the cytosol of nerve terminals and are then stored into synaptic vesicles prior to exocytotic release. Cholinergic nerve terminals synthesize and release the neurotransmitter acetylcholine (ACh).
2. Regulation of ChAT
Choline acetyltransferase (ChAT, acetylCoA:choline O -acetyltransferase, EC 2.3.1.6), the biosynthetic enzyme of the neurotransmitter acetylcholine (ACh), is a key marker required for cholinergic neurotransmission in the central and peripheral nervous system.
3. Sorting and trafficking of proteins to synaptic vesicles
There are two populations of secretory vesicles in cholinergic nerve terminals, synaptic vesicles and large-dense core vesicles (LDCV). VAChT is predominantly found in the former ( Gilmor et al., 1996, Weihe et al., 1996, Garzon and Pickel, 2000 ).
4. Localization and trafficking of VAChT
The VAChT is a 12-transmembrane domain protein with N- and C-terminal regions directed to the cytosol. The protein is found in nerve-terminal fields and in neuronal cell bodies of ChAT positive-cells in the nervous system, as it would be expected for a cholinergic specific protein ( Schafer et al., 1998, Roghani et al., 1998 ).
5. Regulation of VAChT
The possibility of regulation of quantal size (i.e. the amount of neurotransmitter in a single vesicle) due to changes in the activity of VAChT was raised by Van der Kloot and others ( Van der Kloot, 1991, Van der Kloot and Branisteanu, 1992, Van der Kloot and Molgo, 1994 ).
6. Regulation of neurotransmitter storage by vesicular transporters
The level of expression of vesicular transporters has a great potential to influence transmitter release. Over-expression of VAChT in immature xenopus neurons increases the amount of neurotransmitters released by synaptic vesicles ( Song et al., 1997 ).
How long after denervation do cholinoreceptors contract?
This chapter reviews non-synaptic cholinoreceptors At the moment of birth, the skeletal muscles of the rabbit are sensitive to acetylcholine (Ach) over their entire surface area; but in the first days of postnatal ontogenesis the cholinoreceptive zone rapidly contracts, and in 9–10 days becomes restricted, as in the adult animal, solely to the region of entry of the nerve. The reverse process occurs after denervation: the cholinoreceptive zone expands anew from the neural region toward the tendinous ends and embraces the entire surface of the fiber. The data obtained in Ginetsinsky's laboratory has been confirmed in investigations using microelectrodes. In 1 to 2 weeks after denervation the muscle fibers become as sensitive over their entire surface to the electrophoretic application of ACh as the endplate region. The sensitivity of the endplate cholinoreceptors is unaltered after denervation. It is precisely this huge increase in the cholinoreceptive surface, the increase in the total number of ChRs, that explains the ability of denervated muscle to react with a contracture to immersion in a solution of ACh. The action of ACh on each individual ChR evokes the same change in membrane permeability in the denervated muscle as in the normal muscle.
What is the acetylcholine molecule?
Acetylcholine: An Approach to the Molecular Mechanism of Action is an in-depth study of neurotransmitter system, with much focus on acetylcholine and its action and the cholinergic synapse. The book, divided into seven chapters, covers the following topics: the function of the cholinergic synapse; the movement of ions across membranes; the excitatory postsynaptic potential; the nature of cholinoreceptors and cholinesterases; the structure of its active centers; and the pattern of arrangement of the receptors of the cholinoreceptive membrane. The book also covers the quantitative evaluation of the action of neurotransmitters; the reactive capacity of the acetylcholine molecule and its effects on nerve endings and nerve fibers; and the release of mediators and hormones. The text is recommended for those who specialize in the fields of biology, medicine, biochemistry, and pharmacology, especially those wish to study about the neurotransmitter system, cholinoreceptors, the cholinergic synapse, the acetylcholine, and its action.
How are the cholinoreceptive properties of the postsynaptic membrane determined?
The cholinoreceptive properties of the postsynaptic membrane are determined not only by the structure of the individual ChR and to the density of the ChRs, that is, the number of ChRs per unit of membrane surface, but also by the pattern of arrangement of the individual ChRs on the cholinoreceptive membrane.
What enzyme releases ACh into the synaptic cleft?
The termination of the action of the ACh liberated into the synaptic cleft is effected in vertebrates mainly by the enzyme acetylcholinesterase (AChE), which hydrolyses ACh to the physiologically relatively inactive choline and acetic acid.
What is the chemical reaction that occurs on the ChE with ACh?
A chemical reaction occurs on the ChE with ACh, namely, the acetylation of the hydroxyl group of the amino-acid, ser ine, which forms a part of the corresponding active group of ChE; the hydrolysis of ACh is brought about, and this active group of ChE is generally called the esteratic site.
Which enzyme catalyzes the transport of the acetyl residue from coenzyme A to?
Synapses in which acetylcholine (ACh) plays the role of mediator is called cholinergic. ACh is synthesized in the nerve cell with the help of coenzyme A and the specific enzyme choline acetylase, which catalyzes the transport of the acetyl residue from coenzyme A to the choline. Choline acetylase is dissolved in the cytoplasm.
What is acetylcholine: an approach to the Molecular Mechanism of Action?
Acetylcholine: An Approach to the Molecular Mechanism of Action is an in-depth study of neurotransmitter system, with much focus on acetylcholine and its action and the cholinergic ... read full description.