
How to calculate electric potential?
Section Summary
- Electric potential is potential energy per unit charge.
- The potential difference between points A and B, VB − VA, defined to be the change in potential energy of a charge q moved from A to B, is equal ...
- An electron volt is the energy given to a fundamental charge accelerated through a potential difference of 1 V. ...
How to find the electric potential?
Key Points
- The electric potential V is a scalar and has no direction, whereas the electric field E is a vector.
- To find the voltage due to a combination of point charges, you add the individual voltages as numbers. ...
- To find the total electric field, you must add the individual fields as vectors, taking magnitude and direction into account. ...
What is the formula for electric potential?
Solved Examples on Electric Potential
- Solution: Therefore, acceleration = F/m ∝ 1/r 2 i.e., acceleration is not constant during motion. ...
- Solution: Let u be the velocity of the ball at point A. This appears in from of the increased kinetic energy.
- Solution: Given that, the magnitude of charges are q 1 = 1C and q 2 = 2C. ...
- Solution: Therefore, ∆E = 27 × 1010. ...
How to solve for electric potential?
- V = electric potential energy
- q = point charge
- r = distance between any point around the charge to the point charge
- k = Coulomb constant; k = 9.0 × 10 9 N

What is electric potential?
The electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined as the amount of work energy needed to move a unit of electric charge from a reference point to the specific point in an electric field. More precisely, it is the energy per unit charge for a test charge that is so small that the disturbance of the field under consideration is negligible. Furthermore, the motion across the field is supposed to proceed with negligible acceleration, so as to avoid the test charge acquiring kinetic energy or producing radiation. By definition, the electric potential at the reference point is zero units. Typically, the reference point is earth or a point at infinity, although any point can be used.
Which law states that the electric field points downhill towards lower voltages?
This states that the electric field points "downhill" towards lower voltages. By Gauss's law , the potential can also be found to satisfy Poisson's equation :
What is the electrostatic field?
In classical electrostatics, the electrostatic field is a vector quantity which is expressed as the gradient of the electrostatic potential, which is a scalar quantity denoted by V or occasionally φ, equal to the electric potential energy of any charged particle at any location (measured in joules) divided by the charge of that particle (measured in coulombs ). By dividing out the charge on the particle a quotient is obtained that is a property of the electric field itself. In short, electric potential is the electric potential energy per unit charge.
How is the electric field expressed?
Instead, the electric field can be expressed in terms of both the scalar electric potential and the magnetic vector potential. The electric potential and the magnetic vector potential together form a four vector, so that the two kinds of potential are mixed under Lorentz transformations .
What is Coulomb potential?
VE is known as the Coulomb potential . The electric potential for a system of point charges is equal to the sum of the point charges' individual potentials. This fact simplifies calculations significantly, because addition of potential (scalar) fields is much easier than addition of the electric (vector) fields.
How does an electric field exert a force on an object?
If the charged object has a positive charge the force will be in the direction of the electric field vector at that point while if the charge is negative the force will be in the opposite direction. The magnitude of the force is given by the quantity of the charge multiplied by the magnitude of the electric field vector.
How are potential energy and force related?
Classical mechanics explores concepts such as force, energy, and potential. Force and potential energy are directly related. A net force acting on any object will cause it to accelerate. As an object moves in the direction in which the force accelerates it, its potential energy decreases. For example, the gravitational potential energy of a cannonball at the top of a hill is greater than at the base of the hill. As it rolls downhill its potential energy decreases, being translated to motion, kinetic energy.
What is electric potential?
Electric potential is the amount of potential energy per unit of charge.
How does gravitational potential energy work?
A gravitational field exists about the Earth that exerts gravitational influences upon all masses located in the space surrounding it. Moving an object upward against the gravitational field increase s its gravitational potential energy. An object moving downward within the gravitational field would lose gravitational potential energy. When gravitational potential energy was introduced in Unit 5 of The Physics Classroom, it was defined as the energy stored in an object due to its vertical position above the Earth. The amount of gravitational potential energy stored in an object depended upon the amount of mass the object possessed and the amount of height to which it was raised. Gravitational potential energy depended upon object mass and object height. An object with twice the mass would have twice the potential energy and an object with twice the height would have twice the potential energy. It is common to refer to high positions as high potential energy locations. A glance at the diagram at the right reveals the fallacy of such a statement. Observe that the 1 kg mass held at a height of 2 meters has the same potential energy as a 2 kg mass held at a height of 1 meter. Potential energy depends upon more than just location; it also depends upon mass. In this sense, gravitational potential energy depends upon at least two types of quantities:
When work is done on a positive test charge to move it from one location to another, what happens?
When work is done on a positive test charge to move it from one location to another, potential energy increases and electric potential increases.
How does an electric circuit work?
In the electrochemical cells of a battery-powered electric circuit, the chemical energy is used to do work on a positive test charge to move it from the low potential terminal to the high potential terminal. Chemical energy is transformed into electric potential energy within the internal circuit (i.e., the battery). Once at the high potential terminal, a positive test charge will then move through the external circuit and do work upon the light bulb or the motor or the heater coils, transforming its electric potential energy into useful forms for which the circuit was designed. The positive test charge returns to the negative terminal at a low energy and low potential, ready to repeat the cycle (or should we say circuit) all over again.
What is electric potential?
Electric potential, the amount of work needed to move a unit charge from a reference point to a specific point against an electric field. Typically, the reference point is Earth, although any point beyond the influence of the electric field charge can be used. The electric potential is another useful field.
How is electric potential measured?
Although the concept of electric potential is useful in understanding electricalphenomena, only differences in potential energy are measurable. If an electric field is defined as the force per unit charge, then by analogyan electric potential can be thought of as the potential energy per unit charge. Therefore, the work done in moving a unit charge from one point to another (e.g., within an electric circuit) is equal to the difference in potential energies at each point. In the International System of Units(SI), electric potential is expressed in units of joulesper coulomb(i.e., volts), and differences in potential energy are measured with a voltmeter.
What is the difference between conductometry and potentiometry?
Potentiometry measures electric potential(or voltage) while maintaining a constant (normally nearly zero) electric current between the electrodes. Amperometry monitors electric current (amperes) while keeping the potential constant. Conduct ometry measures conductance (the ability of a solution to carry an electric current) while a constant alternating-current (AC) potential…
Is electric potential a scalar function?
The electric potentialis just such a scalar function. Electric potential is related to the work done by an external force when it transports a charge slowly from one position to another in an environment containing other charges at rest. The difference between…
Does potential energy depend on the path taken?
The potential energy for a positive charge increases when it moves against an electric field and decreases when it moves with the electric field; the opposite is true for a negative charge. Unless the unit charge crosses a changing magnetic field, its potential at any given point does not depend on the path taken.
Is electric potential a field?
The electric potentialis another useful field. It provides an alternative to the electric field in electrostatics problems. The potential is easier to use, however, because it is a single number, a scalar, instead of a vector. The difference in potential between two places measures the…
What is the probability distribution of electrons?
When in the area around the nucleus of an atom, electrons have a certain probability distribution described by the Schrödinger equation, meaning that in some spots there is a higher chance of spotting an electron than in others (as shown in the image). Interestingly, this distribution actually means there is a possibility of the electrons going inside the nucleus.
How does potential energy decrease?
The potential energy decreases due to release of that energy as light or photons mostly. This is the basis of color that we see in reference to visible light. All types of EM waves can originate from within the atoms with electrons falling from outer shells to inner shells and releasing the commensurate amount of their potential energy as photons, many of which will be in the visible spectrum. The Planck-Einstein equation for the energy of the photon released is : E = hf, where f is the frequency of light which determines the color of light. So in short an electron coming closer to the nucleus
How can electrons be studied?
The behavior of electron can be studied through it's wave function which is solution of Schrodinger equation with admissibility ( boundary) conditions. These conditions lead to quantization of energy eigen values of Schrodinger equation . Now, the electrons can exist in various stable quantum states with different probabilities .
How long does an electron have to live in a quantum state?
Electron in high energy quantum state has life time~ 10^-8s and makes spontaneous transitions to lower energy state emitting the photon of energy equal to difference of energy between two states.
Why doesn't the probability sphere concentrate more around the positive charge of the nucleus?
The reason this probability sphere doesn't concentrate more around the positive charge of the nucleus is because of another consequence of the uncertainty principle: the more accurately you determine the position of an electron, the less accurately you can determine its momentum, and vice versa. If the probability distribution was concentrated around the nucleus, the mean square of the momentum would become much larger [ 1], meaning the forces keeping the electrons around the nucleus won't be high enough to stop the electrons "flying off".
What energy is stored in an electron?
The energy we are taking about is Potential Energy that gets stored in Electron due to the attraction force by nucleus.
Does an electron exist inside the nucleus?
At first, electron does not exist inside the nucleus.
What is the difference between electrochemical potential and electrochemical potential?
In electrochemistry, the electrochemical potential of electrons (or any other species) is the total potential, including both the (internal, nonelectrical) chemical potential and the electric potential , and is by definition constant across a device in equilibrium, whereas the chemical potential of electrons is equal to the electrochemical potential minus the local electric potential energy per electron. In solid-state physics, the definitions are normally compatible with this, but occasionally the definitions are swapped.
Why is electrochemical potential important?
Electrochemical potential is important in biological processes that involve molecular diffusion across membranes, in electroanalytical chemistry, and industrial applications such as batteries and fuel cells. It represents one of the many interchangeable forms of potential energy through which energy may be conserved .
What is the electrode potential of corroding metals called?
In some contexts, the electrode potential of corroding metals is called "electrochemical corrosion potential", which is often abbreviated as ECP, and the word "corrosion" is sometimes omitted. This usage can lead to confusion.
What happens to the electrochemical potential of a species?
If possible, a species will move from areas with higher electrochemical potential to areas with lower electrochemical potential; in equilibrium, the electrochemical potential will be constant everywhere for each species (it may have a different value for different species). For example, if a glass of water has sodium ions (Na +) ...
What is the mechanical work done in bringing 1 mole of an ion from a standard state to?
In generic terms, electrochemical potential is the mechanical work done in bringing 1 mole of an ion from a standard state to a specified concentration and electrical potential . According to the IUPAC definition, it is the partial molar Gibbs energy of the substance at the specified electric potential, where the substance is in a specified phase. Electrochemical potential can be expressed as
What is the ECP in electrochemistry?
In electrochemistry, the electrochemical potential ( ECP ), μ, is a thermodynamic measure of chemical potential that does not omit the energy contribution of electrostatics. Electrochemical potential is expressed in the unit of J / mol .
What is the sum of the chemical potential and the membrane potential?
In cell membranes, the electrochemical potential is the sum of the chemical potential and the membrane potential .
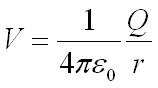
Overview
The electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined as the amount of work energy needed to move a unit of electric charge from a reference point to the specific point in an electric field. More precisely, it is the energy per unit charge for a test charge that is so small that the disturbance of the field under consideration is negligible. Furthermore, the motion across the field is supposed to proceed with negligible accel…
Introduction
Classical mechanics explores concepts such as force, energy, and potential. Force and potential energy are directly related. A net force acting on any object will cause it to accelerate. As an object moves in the direction of the force that is acting, its potential energy decreases. For example, the gravitational potential energy of a cannonball at the top of a hill is greater than at the base of the hill. As it rolls downhill, its potential energy decreases and is being translated to motion - kinetic …
Electrostatics
The electric potential at a point r in a static electric field E is given by the line integral
where C is an arbitrary path from some fixed reference point to . In electrostatics, the Maxwell-Faraday equation reveals that the curl is zero, making the electric field conservative. Thus, the line integral above does not depend on the specific path C chosen but only on its endpoints, making well-defined everywhere. The gradient theorem then allows us to write:
Generalization to electrodynamics
When time-varying magnetic fields are present (which is true whenever there are time-varying electric fields and vice versa), it is not possible to describe the electric field simply in terms of a scalar potential V because the electric field is no longer conservative: is path-dependent because (due to the Maxwell-Faraday equation).
Instead, one can still define a scalar potential by also including the magnetic vector potential A. I…
Units
The SI derived unit of electric potential is the volt (in honor of Alessandro Volta), which is why a difference in electric potential between two points is known as voltage. Older units are rarely used today. Variants of the centimetre–gram–second system of units included a number of different units for electric potential, including the abvolt and the statvolt.
Galvani potential versus electrochemical potential
Inside metals (and other solids and liquids), the energy of an electron is affected not only by the electric potential, but also by the specific atomic environment that it is in. When a voltmeter is connected between two different types of metal, it measures the potential difference corrected for the different atomic environments. The quantity measured by a voltmeter is called electrochemical potential or fermi level, while the pure unadjusted electric potential V is sometimes called Galvani …
See also
• Absolute electrode potential
• Electrochemical potential
• Electrode potential
Further reading
• Politzer P, Truhlar DG (1981). Chemical Applications of Atomic and Molecular Electrostatic Potentials: Reactivity, Structure, Scattering, and Energetics of Organic, Inorganic, and Biological Systems. Boston, MA: Springer US. ISBN 978-1-4757-9634-6.
• Sen K, Murray JS (1996). Molecular Electrostatic Potentials: Concepts and Applications. Amsterdam: Elsevier. ISBN 978-0-444-82353-3.