Applications of Photoelectric Effect
- (1) In the construction of solar cell, which is a non-conventional energy source (highly required by mankind in the present world).
- (2) In photo-telegraphy, whereby the help of photo-tubes, the light and shades of pictures are converted into electrical waves which are transmitted to distant stations.
- (3) The reproduction of sound in cinema films. ...
What is the energy band of an atom?
How does photoconductivity occur?
What is the energy of a photon?
How does illumination affect photoelectric energy?
How is voltage generated in photovoltaics?
What happens when an inner electron is ejected?
What is the photoelectric effect?
See 4 more
About this website
What is a real life example of the photoelectric effect?
Applications of the photoelectric effect brought us "electric eye" door openers, light meters used in photography, solar panels and photostatic copying.
Where is photoelectric effect found?
The photoelectric effect was discovered in 1887 by the German physicist Heinrich Rudolf Hertz. In connection with work on radio waves, Hertz observed that, when ultraviolet light shines on two metal electrodes with a voltage applied across them, the light changes the voltage at which sparking takes place.
What is photoelectric effect give an example?
Examples of photoelectric effects are : 1.When light shines on a cathode plate, the emitted electrons from the plate hit the anode and create a current. A solar panel is created from linking these together. 2.
Why is photoelectric effect important?
The photoelectric effect is significant because it demonstrates that light has particle-like qualities. It established that we can consider light as photons (packets) of energy where one photon interacts w/ one electron and each photon must have sufficient energy to remove each electron.
Do solar panels use photoelectric effect?
Photovoltaic solar energy is generated by converting sunlight into energy, a type of clean, renewable, and inexhaustible energy that can be produced in installations ranging from small panels on the top of houses to large photovoltaic plants. This is achieved using a technology based on the photoelectric effect.
Does the photoelectric effect only work on metals?
Does the photoelectric effect take place only in metals? Photoelectric effect can take place in any materials. Though it is more commonly associated with alkali metals, it's also quite important with semiconductors.
What is the photoelectric effect for dummies?
Introduction: What is the photoelectric effect? When light shines on a metal, electrons can be ejected from the surface of the metal in a phenomenon known as the photoelectric effect. This process is also often referred to as photoemission, and the electrons that are ejected from the metal are called photoelectrons.
How did Albert Einstein explain the photoelectric effect?
Light, Einstein said, is a beam of particles whose energies are related to their frequencies according to Planck's formula. When that beam is directed at a metal, the photons collide with the atoms. If a photon's frequency is sufficient to knock off an electron, the collision produces the photoelectric effect.
Does the photoelectric effect only work on metals?
Does the photoelectric effect take place only in metals? Photoelectric effect can take place in any materials. Though it is more commonly associated with alkali metals, it's also quite important with semiconductors.
How is the photoelectric effect used in the medical field?
The photoelectric effect is the most important effect in medical radiography. E.g. it is photoelectric absorption that is responsible for most of the absorption in a mammogram which creates the contrast in the image. See also Photon, Electron.
What is photoelectric cell in physics?
photoelectric cell, also called Electric Eye, Photocell, or Phototube, an electron tube with a photosensitive cathode that emits electrons when illuminated and an anode for collecting the emitted electrons.
How was photoelectric effect discovered?
Heinrich Hertz, a German physicist, discovered the photoelectric effect in 1887. He observed that shining an ultraviolet light on electrodes caused a change in the voltage between them. Other work during the 19th century built on Hertz's observations.
What are the conditions for the photoelectric effect?
The minimum condition required for the emission of electrons from the outermost shell of an atom is that the frequency of incident rays should be v...
What is the importance of the photoelectric effect?
The study of the photoelectric effect has led to expanding our understanding of the quantum nature of light and electrons. It has further influence...
Who discovered the photoelectric effect?
The phenomenon of the photoelectric effect was discovered by Heinrich Hertz in 1887.
Which popular device is based on the photoelectric effect?
One of the common devices based on this effect is the photoelectric cell or photodiode.
What are some examples of photoelectric effect? | Socratic
Solar panels! When light shines on a cathode plate, the emitted electrons from the plate hit the anode and create a current. A solar panel is created from linking these together. Source, with a few more examples. Also, there was a case where raspberry pis, which are very small computers often used by hobbyists, would shut down when someone took a picture of it.
Photoelectric Effect: Definition, Examples and Applications - Collegedunia
Histroy of Photoelectric Effect [Click Here for Sample Questions] Wilhelm Ludwig Franz Hallwachs propounded the idea of the Photoelectric Effect in 1887, followed by Heinrich Rudolf Hertz proving the phenomenon through experiments.
Photoelectric Effect Formula: Meaning, Formula, Examples - Toppr-guides
A photon particle is the tiny blob of pure energy. Under suitable circumstances, we can use light to push electrons and free them from the surface of a solid. This process is termed as the photoelectric effect or photoelectric emission. This article will explain the photoelectric effect formula and examples.
Photoelectric Effect - Definition, Equation, Characteristics ...
For λ = wavelength of the incident photon, then. If λ<λ th, the photoelectric effect will occur, and the expelled electron will have kinetic energy.; If λ= λ th, the photoelectric effect will be the only one that occurs, and the kinetic energy of the ejected photoelectron will be zero.; There will be no photoelectric effect if λ>λ th.; Work Function or Threshold Energy (Φ): The work ...
What is the photoelectric effect?
The photoelectric effect is a phenomenon in which electrons are ejected from the surface of a metal when light is incident on it. These ejected electrons are called photoelectrons. It is important to note that the emission of photoelectrons and the kinetic energy of the ejected photoelectrons is dependent on the frequency ...
Why does the photoelectric effect not occur when the red light strikes the metallic surface?
The photoelectric effect does not occur when the red light strikes the metallic surface because the frequency of red light is lower than the threshold frequency of the metal. The photoelectric effect occurs when green light strikes the metallic surface and photoelectrons are emitted. The photoelectric effect also occurs when blue light strikes ...
What happens if the energy of a photon is less than the threshold energy?
If the energy of the photon is less than the threshold energy, there will be no emission of photoelectrons (since the attractive forces between the nuclei and the electrons cannot be overcome). Thus, the photoelectric effect will not occur if 𝜈 < 𝜈 th.
What is the term for the phenomenon of metals releasing electrons when they are exposed to the light of the appropriate?
Definition of Photoelectric Effect. The phenomenon of metals releasing electrons when they are exposed to the light of the appropriate frequency is called the photoelectric effect, and the electrons emitted during the process are called photoelectrons.
What is the term for the light that is ejected from a metal?
To be more precise, light incident on the surface of a metal in the photoelectric effect causes electrons to be ejected. The electron ejected due to the photoelectric effect is called a photoelectron and is denoted by e –. The current produced as a result of the ejected electrons is called photoelectric current.
How does photoelectric energy work?
For the photoelectric effect to occur, the photons that are incident on the surface of the metal must carry sufficient energy to overcome the attractive forces that bind the electrons to the nuclei of the metals . The minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the symbol Φ). For a photon to possess energy equal to the threshold energy, its frequency must be equal to the threshold frequency (which is the minimum frequency of light required for the photoelectric effect to occur). The threshold frequency is usually denoted by the symbol 𝜈 th and the associated wavelength (called the threshold wavelength) is denoted by the symbol λ th. The relationship between the threshold energy and the threshold frequency can be expressed as follows.
What is the threshold wavelength of a photoelectric effect?
1. In a photoelectric effect experiment, the threshold wavelength of incident light is 260 nm and E (in eV) = 1237/λ (nm). Find the maximum kinetic energy of emitted electrons.
How did Einstein explain the photoelectric effect?
Albert Einstein's mathematical description of how the photoelectric effect was caused by absorption of quanta of light was in one of his Annus Mirabilis papers, named " On a Heuristic Viewpoint Concerning the Production and Transformation of Light ". The paper proposed a simple description of light quanta, or photons, and showed how they explained such phenomena as the photoelectric effect. His simple explanation in terms of absorption of discrete quanta of light agreed with experimental results. It explained why the energy of photoelectrons was dependent only on the frequency of the incident light and not on its intensity: at low-intensity, the high-frequency source could supply a few high energy photons, whereas at high-intensity, the low-frequency source would supply no photons of sufficient individual energy to dislodge any electrons. This was an enormous theoretical leap, but the concept was strongly resisted at first because it contradicted the wave theory of light that followed naturally from James Clerk Maxwell 's equations of electromagnetism, and more generally, the assumption of infinite divisibility of energy in physical systems. Even after experiments showed that Einstein's equations for the photoelectric effect were accurate, resistance to the idea of photons continued.
Why is photoemission a process?
This is because the process produces a charge imbalance which, if not neutralized by current flow, results in the increasing potential barrier until the emission completely ceases. The energy barrier to photoemission is usually increased by nonconductive oxide layers on metal surfaces, so most practical experiments and devices based on the photoelectric effect use clean metal surfaces in evacuated tubes. Vacuum also helps observing the electrons since it prevents gases from impeding their flow between the electrodes.
What is the inner photoelectric effect?
Inner photoelectric effect in the bulk of the material that is a direct optical transition between an occupied and an unoccupied electronic state. This effect is subject to quantum-mechanical selection rules for dipole transitions. The hole left behind the electron can give rise to secondary electron emission, or the so-called Auger effect, which may be visible even when the primary photoelectron does not leave the material. In molecular solids phonons are excited in this step and may be visible as satellite lines in the final electron energy.
What is the classical setup to observe the photoelectric effect?
The classical setup to observe the photoelectric effect includes a light source, a set of filters to monochromatize the light, a vacuum tube transparent to ultraviolet light, an emitting electrode (E) exposed to the light, and a collector (C) whose voltage VC can be externally controlled.
How does light affect electrons?
Emission of conduction electrons from typical metals requires a few electron-volt (eV) light quanta, corresponding to short-wavelength visible or ultraviolet light. In extreme cases, emissions are induced with photons approaching zero energy, like in systems with negative electron affinity and the emission from excited states, or a few hundred keV photons for core electrons in elements with a high atomic number. Study of the photoelectric effect led to important steps in understanding the quantum nature of light and electrons and influenced the formation of the concept of wave–particle duality. Other phenomena where light affects the movement of electric charges include the photoconductive effect, the photovoltaic effect, and the photoelectrochemical effect .
When no additional photoelectrons can be collected, the photoelectric current attains a saturation value?
When no additional photoelectrons can be collected, the photoelectric current attains a saturation value. This current can only increase with the increase of the intensity of light. An increasing negative voltage prevents all but the highest-energy electrons from reaching the collector.
How are electronic properties of ordered crystalline solids determined?
The electronic properties of ordered, crystalline solids are determined by the distribution of the electronic states with respect to energy and momentum— the electronic band structure of the solid. Theoretical models of photoemission from solids show that this distribution is, for the most part, preserved in the photoelectric effect. The phenomenological three-step model for ultraviolet and soft X-ray excitation decomposes the effect into these steps:
How does light behave under photoelectric effect?
According to Einstein, light is made up of little packets, at first called quanta and later photons. How quanta behave under the photoelectric effect can be understood through a thought experiment. Imagine a marble circling in a well, which would be like a bound electron to an atom. When a photon comes in, it hits the marble (or electron), giving it enough energy to escape from the well. This explains the behavior of light striking metal surfaces.
What are the applications of photodiodes?
Other applications of photodiodes and photomultipliers include: 1 imaging technology, including (older) television camera tubes or image intensifiers; 2 studying nuclear processes; 3 chemically analyzing materials based on their emitted electrons; 4 giving theoretical information about how electrons in atoms transition between different energy states.
What is the photoelectric effect?
The photoelectric effect refers to what happens when electrons are emitted from a material that has absorbed electromagnetic radiation. Physicist Albert Einstein was the first to describe the effect fully, and received a Nobel Prize for his work.
What is the purpose of photoelectric cells?
Photoelectric cells were originally used to detect light, using a vacuum tube containing a cathode, to emit electrons, and an anode, to gather the resulting current . Today, these "phototubes" have advanced to semiconductor-based photodiodes that are used in applications such as solar cells and fiber optics telecommunications.
What was the most important application of the photoelectric effect?
But perhaps the most important application of the photoelectric effect was setting off the quantum revolution, according to. Scientific American. It led physicists to think about the nature of light and the structure of atoms in an entirely new way.
What happens when a photon hits a marble?
When a photon comes in, it hits the marble (or electron), giving it enough energy to escape from the well. This explains the behavior of light striking metal surfaces. While Einstein, then a young patent clerk in Switzerland, explained the phenomenon in 1905, it took 16 more years for the Nobel Prize to be awarded for his work.
What is the particle that collides with an electron called?
Each particle of light, called a photon, collides with an electron and uses some of its energy to dislodge the electron. The rest of the photon's energy transfers to the free negative charge, called a photoelectron. Understanding how this works revolutionized modern physics. Applications of the photoelectric effect brought us "electric eye" door ...
What is the energy band of an atom?
The highest energy configuration (or energy band) that is normally occupied by electrons for a given material is known as the valence band , and the degree to which it is filled largely determines the material’s electrical conductivity. In a typical conductor (metal), the valence band is about half filled with electrons, which readily move from atom to atom, carrying a current. In a good insulator, such as glass or rubber, the valence band is filled, and these valence electrons have very little mobility. Like insulators, semiconductors generally have their valence bands filled, but, unlike insulators, very little energy is required to excite an electron from the valence band to the next allowed energy band—known as the conduction band, because any electron excited to this higher energy level is relatively free. For example, the “bandgap” for silicon is 1.12 eV ( electron volts ), and that of gallium arsenide is 1.42 eV. This is in the range of energy carried by photons of infrared and visible light, which can therefore raise electrons in semiconductors to the conduction band. (For comparison, an ordinary flashlight battery imparts 1.5 eV to each electron that passes through it. Much more energetic radiation is required to overcome the bandgap in insulators.) Depending on how the semiconducting material is configured, this radiation may enhance its electrical conductivity by adding to an electric current already induced by an applied voltage ( see photoconductivity ), or it may generate a voltage independently of any external voltage sources ( see photovoltaic effect ).
How does photoconductivity occur?
Photoconductivity arises from the electrons freed by the light and from a flow of positive charge as well. Electrons raised to the conduction band correspond to missing negative charges in the valence band, called “holes.”. Both electrons and holes increase current flow when the semiconductor is illuminated.
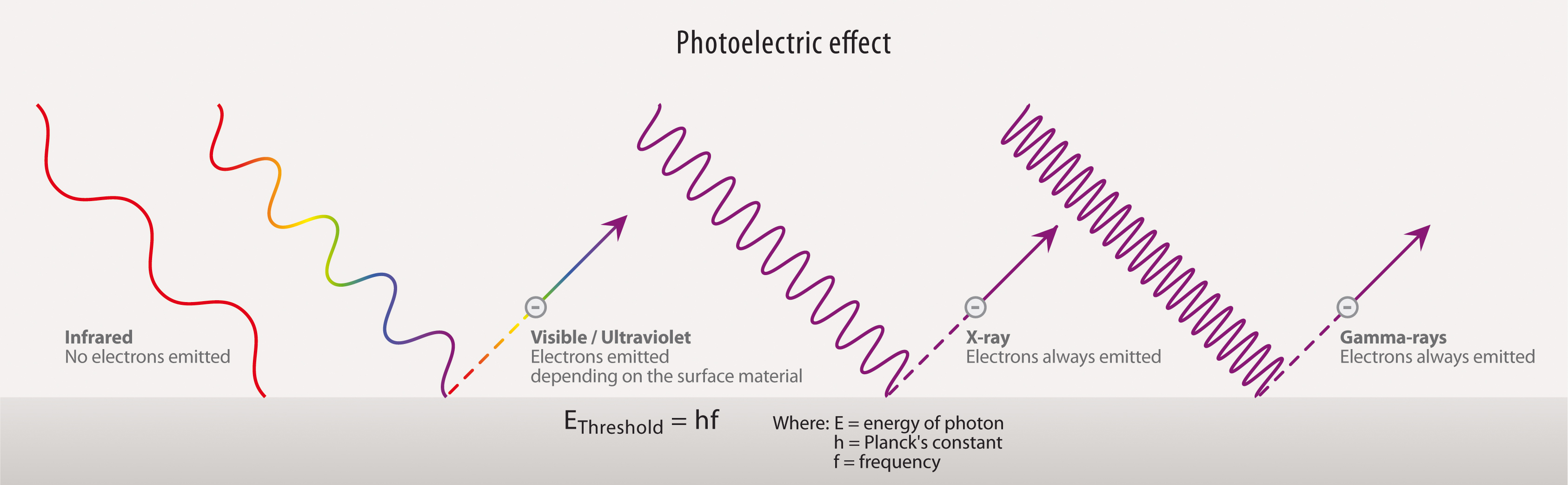
What is the energy of a photon?
Consideration of these unexpected behaviours led Albert Einstein to formulate in 1905 a new corpuscular theory of light in which each particle of light, or photon, contains a fixed amount of energy, or quantum, that depends on the light’s frequency. In particular, a photon carries an energy E equal to hf, where f is the frequency of the light and h is the universal constant that the German physicist Max Planck derived in 1900 to explain the wavelength distribution of blackbody radiation—that is, the electromagnetic radiation emitted from a hot body. The relationship may also be written in the equivalent form E = hc /λ, where c is the speed of light and λ is its wavelength, showing that the energy of a photon is inversely proportional to its wavelength.
How does illumination affect photoelectric energy?
Other photoelectric effects are caused by radiation at higher frequencies, such as X-rays and gamma rays.
How is voltage generated in photovoltaics?
In the photovoltaic effect, a voltage is generated when the electrons freed by the incident light are separated from the holes that are generated , producing a difference in electrical potential. This is typically done by using a p - n junction rather than a pure semiconductor.
What happens when an inner electron is ejected?
When such an inner electron is ejected, a higher-energy outer electron quickly drops down to fill the vacancy. The excess energy results in the emission of one or more additional electrons from the atom, which is called the Auger effect.
What is the photoelectric effect?
photoelectric effect, phenomenon in which electrically charged particles are released from or within a material when it absorbs electromagnetic radiation. The effect is often defined as the ejection of electrons from a metal plate when light falls on it. In a broader definition, the radiant energy may be infrared, visible, or ultraviolet light, ...

Overview
The photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, and solid state and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in electronic devices sp…
Emission mechanism
The photons of a light beam have a characteristic energy, called photon energy, which is proportional to the frequency of the light. In the photoemission process, when an electron within some material absorbs the energy of a photon and acquires more energy than its binding energy, it is likely to be ejected. If the photon energy is too low, the electron is unable to escape the material. Since a…
History
In 1839, Alexandre Edmond Becquerel discovered the photovoltaic effect while studying the effect of light on electrolytic cells. Though not equivalent to the photoelectric effect, his work on photovoltaics was instrumental in showing a strong relationship between light and electronic properties of materials. In 1873, Willoughby Smith discovered photoconductivity in selenium while testing the me…
Uses and effects
These are extremely light-sensitive vacuum tubes with a coated photocathode inside the envelope. The photo cathode contains combinations of materials such as cesium, rubidium, and antimony specially selected to provide a low work function, so when illuminated even by very low levels of light, the photocathode readily releases electrons. By means of a series of electrodes (d…
Competing processes and photoemission cross section
When photon energies are as high as the electron rest energy of 511 keV, yet another process, the Compton scattering, may take place. Above twice this energy, at 1.022 MeV pair production is also more likely. Compton scattering and pair production are examples of two other competing mechanisms.
Even if the photoelectric effect is the favoured reaction for a particular interaction of a single ph…
External links
• Astronomy Cast "http://www.astronomycast.com/2014/02/ep-335-photoelectric-effect/". AstronomyCast.
• Nave, R., "Wave-Particle Duality". HyperPhysics.
• "Photoelectric effect". Physics 2000. University of Colorado, Boulder, Colorado. (page not found)