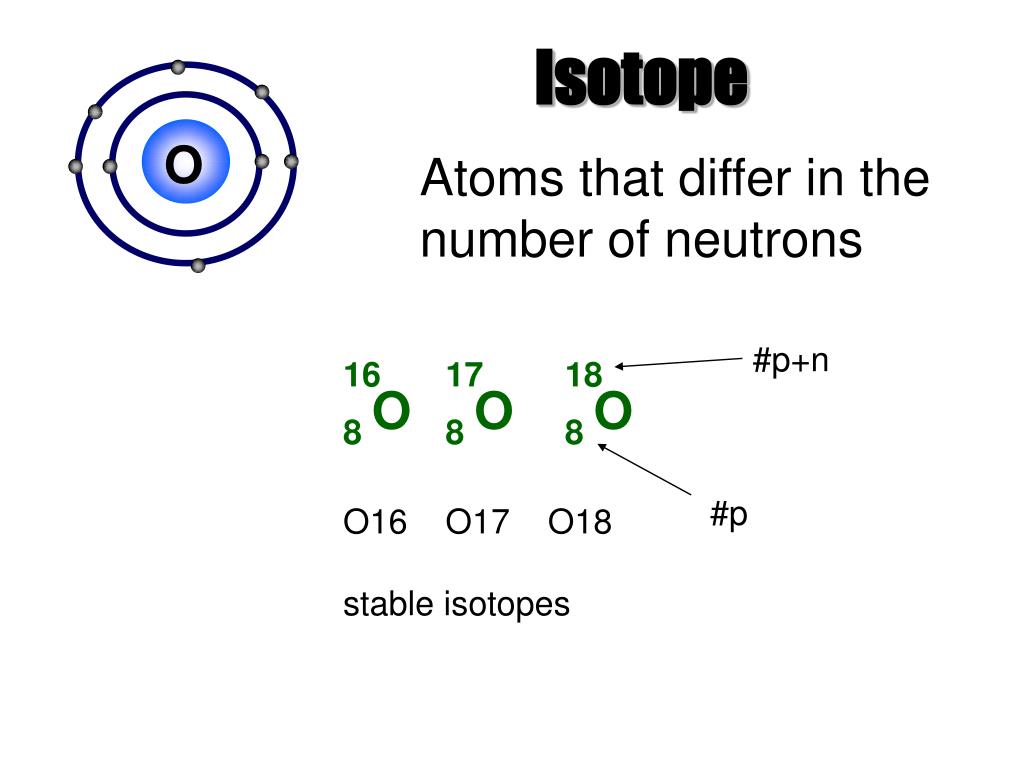
The lighter isotopes mostly decay to isotopes of sodium while the heavier isotopes decay to isotopes of aluminium. Magnesium-24 is composed of 12 protons, 12 neutrons, and 12 electrons. Magnesium-25 is composed of 12 protons, 13 neutrons, and 12 electrons. Magnesium-26 is composed of 12 protons, 12 neutrons, and 12 electrons.
Full Answer
How many protons neutrons and electrons are in a magnesium atom?
The atomic number of magnesium is 12 and it has 12 protons and 12 neutrons in it’s nucleus. Mg is the second most abundant element in the universe, after hydrogen. How many neutrons are in an atom of magnesium 22? How do you find protons neutrons and electrons? How do you find number of protons? What has 12 protons and 12 neutrons?
What are isotopes of magnesium?
Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. Mass numbers of typical isotopes of Magnesium are 24; 25 ;26. Magnesium naturally occurs in three stable isotopes, 24Mg, 25Mg, and 26Mg.
What is the atomic number of magnesium?
Magnesium is the 12th element of the periodic table so its atomic number is 12. The atomic number of an element is equal to the number of protons and electrons in that element. Therefore, a magnesium atom has twelve protons and twelve electrons.
Does the nucleus of an atom contain protons and neutrons?
Experiments by various scientists have shown that the nucleus of an atom contains protons and neutrons. The only exception is hydrogen, which has only protons in its nucleus but no neutrons. Electrons revolve around the nucleus in a specific orbit. How to easily find the number of electrons, protons and neutrons in a magnesium atom?
How many neutrons are in magnesium?
What is the atomic number of magnesium?
What is the fractional abundance of Mg-12?
About this website

What isotope has 12 protons and 12 neutrons?
Magnesium atomsThere are also synthetic isotopes, created as byproducts of nuclear decay or intentionally for commercial use, so they aren't included. So you might account for this isotope problem by saying that about 79% of all Magnesium atoms have 12 neutrons, 12 protons, and 12 electrons.
Which atom is an isotope of magnesium?
Magnesium-25 atom is the stable isotope of magnesium with relative atomic mass 24.985837, 10.0 atom percent natural abundance and nuclear spin 5/2.
What is the atomic number of an atom that contains 12 protons 12 neutrons and 12 electrons?
The number of electrons in an atom is equal to the number of protons of that atom. So, the number of electrons of the atom is 12. The atomic number of an atom is equal to the number of electrons. So, the atomic number is also 12.
What is the atomic mass of magnesium if an atom has 12 protons 12 neutrons and 12 electrons?
24.305 atomic mass unitsNameMagnesiumAtomic Mass24.305 atomic mass unitsNumber of Protons12Number of Neutrons12Number of Electrons129 more rows
What are the two isotopes of magnesium?
Magnesium has two isotopes ^2412Mg and ^2612Mg .
How do you find the isotopes of magnesium?
0:322:54Find the Average Atomic Mass - Example: Magnesium - YouTubeYouTubeStart of suggested clipEnd of suggested clipYou need to do to find the average atomic mass is multiply each of the masses. For example twentyMoreYou need to do to find the average atomic mass is multiply each of the masses. For example twenty three point nine eight five by each of the isotopic abundances.
What is the element with 12 protons?
magnesiumOn the periodic table of elements, magnesium is represented by the symbol Mg. It has an atomic number of 12 because it has 12 protons in its nucleus.
What is the atom has 12 protons 12 electrons and 13 neutrons?
magnesium atomThe charge is the difference in the number of protons compared to the number of electrons. You can read more about charge, protons, and electrons later on. From the example, you can see that this magnesium atom would have 12 protons, 13 neutrons, and 10 electrons.
What is the atomic number of an element with 12 protons 10 neutrons and 12 electrons?
Magnesium Ion has 12 protons and 10 neutrons. Mg and has an atomic number of 12, which means 12 protons are in it.
What is a mass number of 12 protons and 12 neutrons?
Hence atomic number = number of protons= 12 Atomic mass is sum of protons and neutrons present in atom. Therefore mass number = number of protons p + number of neutrons n = 12 + 12 = 24. Related Answer. `Mg^(2+)` का परमाणु क्रमांक 12 है।
What is the atomic number of an element contains 12 neutrons and having a mass number of 24?
MagnesiumExamplesElementMass numberNeutronsMagnesium2412Potassium3920Carbon126
How many electrons does the following atom possess Note atom contains 12 protons and electron charge of 2 )?
10 electrons12 protons, 12 neutrons, 10 electrons There are 10 electrons because 12-2 = 10. There are 12 neutrons because 24-12 = 12.
What is the most common isotope of magnesium?
magnesium-24The most common stable isotope of magnesium has 12 neutrons — particles with a neutral charge — in each nucleus, giving this version of the element an atomic mass of 24. As a result, it's called magnesium-24.
What is MG 25 used for?
Magnesium Isotopes Mg-25 and Mg-26 are used to study the absorption and metabolism of Mg in the human body and they are also used for heart disease studies. Mg-25 is also used for the production of the radioisotope Na-22.
What is the atomic number for magnesium 24?
12Fact boxGroup2650°C, 1202°F, 923 KAtomic number1224.305State at 20°CSolid24MgElectron configuration[Ne] 3s27439-95-4ChemSpider ID4575328ChemSpider is a free chemical structure database2 more rows
How many neutrons are in mg 26?
14 neutronsThey differ only because a 24Mg atom has 12 neutrons in its nucleus, a 25Mg atom has 13 neutrons, and a 26Mg has 14 neutrons.
chapter 3 Flashcards | Quizlet
Study with Quizlet and memorize flashcards containing terms like 1. A sample of ammonia has a mass of 45.5 g. How many molecules are in this sample? a) 2.67 molecules b) 2.74 x 1025 molecules c) 2.25 x 1023 molecules d) 1.61 x 1024 molecules e) 9.02 x 10-16 molecules, 2. How many moles of hydrochloric acid are contained in a 52.6-g sample of this acid?
Chemistry Chapter 3 Flashcards | Quizlet
Study with Quizlet and memorize flashcards containing terms like 1. Bromine exists naturally as a mixture of bromine-79 and bromine-81 isotopes. An atom of bromine-79 contains A) 35 protons, 44 neutrons, 35 electrons. B) 34 protons and 35 electrons, only. C) 44 protons, 44 electrons, and 35 neutrons. D) 35 protons, 79 neutrons, and 35 electrons. E) 79 protons, 79 electrons, and 35 neutrons., 2 ...
quiz 2 answers.pdf - Name: _ Date: _ VERSION: A Chem 11...
View quiz 2 answers.pdf from CHEM 11 at Santa Monica College. Name: _ Date: _ VERSION: A Chem 11 Spring 2020 Quiz 2 1. One molecule of a compound weighs 2.56 10–22 g. The molar mass of this compound
CHEM-113. Problem Set I. Chapter 3 - StuDocu
chap 3 problem set 1 chapter problem set sample of ammonia has mass of 45.5 how many molecules are in this sample? 2.67 molecules 2.74 1025 molecules 2.25 1023
Solved Naturally occurring copper exists in two isotopic - Chegg
Science; Chemistry; Chemistry questions and answers; Naturally occurring copper exists in two isotopic forms: 63 Cu and 65Cu. The atomic mass of copper is 63.55 amu.
How many protons does magnesium have?
Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
How many electrons are in a neutral atom of magnesium?
Therefore, the number of electrons in neutral atom of Magnesium is 12. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
What is electron configuration?
The electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. Every solid, liquid, gas, and plasma is composed ...
How do neutrons stabilize the nucleus?
Neutrons stabilize the nucleus, because they attract each other and protons , which helps offset the electrical repulsion between protons. As a result, as the number of protons increases, an increasing ratio of neutrons to protons is needed to form a stable nucleus. If there are too many or too few neutrons for a given number of protons, the resulting nucleus is not stable and it undergoes radioactive decay . Unstable isotopes decay through various radioactive decay pathways, most commonly alpha decay, beta decay, or electron capture. Many other rare types of decay, such as spontaneous fission or neutron emission are known. It should be noted that all of these decay pathways may be accompanied by the subsequent emission of gamma radiation. Pure alpha or beta decays are very rare.
What is the name of the group 2 element?
Magnesium is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column (group 2, or alkaline earth metals) of the periodic table: all group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure. Magnesium is the third-most-commonly-used ...
What happens when there are too many neutrons in a nucleus?
If there are too many or too few neutrons for a given number of protons, the resulting nucleus is not stable and it undergoes radioactive decay . Unstable isotopes decay through various radioactive decay pathways, most commonly alpha decay, beta decay, or electron capture.
How much does a protons mass equal?
It has a positive electric charge (+1e) and a rest mass equal to 1.67262 × 10 −27 kg ( 938.272 MeV/c 2 )— marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron.
Magnesium isotopes
Isotopes are members of an element's family that have the same number of protons but differ in the number of neutrons they have.
Answer
Mg is read as “magnesium 25,” and can be written as “magnesium-25” or “Mg-25.” All magnesium atoms have 12 protons in their nucleus. They differ only because a 24Mg atom has 12 neutrons in its nucleus, a 25Mg atom has 13 neutrons, and a 26Mg has 14 neutrons.
New questions in Chemistry
If the density of iron is 7.86 g/cm, what would be the mass of an iron rod whose volume is 30 cm?
How many neutrons are in magnesium?
Magnesium has an atomic mass of 24.3. there are two isotopes of magnesium - one contains 12 neutrons and the other contains 13 neutrons in the nucleus. what is the fractional abundance of the one that contains 12 neutrons?
What is the atomic number of magnesium?
Magnesium's atomic number is 12 , so the natural occurring isotope for magnesium is Mg-12 (12 protons and 12 neutrons). Added up we have an atomic mass of 24 amu. Which means if we added one neutron in Mg-13, our atomic mass would be 25 amu.
What is the fractional abundance of Mg-12?
So in this case, the fractional abundance of Mg-12 would be .7, or 70%.
How many protons does magnesium have?
A magnesium (Mg) atom with a mass number of 25 contains#N#1. None of these#N#2. 13 protons, 12 neutrons and 12 electrons.#N#3. 25 protons, 25 neutrons and 25 electrons.#N#4. 12 protons, 13 neutrons and 12 electrons.#N#5. 25 protons, 13 neutrons and 25 electrons.
Which is greater, the mass of a proton or the mass of an electron?
1. The mass of a proton is much greater than the mass of an electron.
Which atoms have the same number of protons?
3. All sodium atoms have the same number of protons.
Where does the positive charge in an atom reside?
2. the positive charge in an atom must reside in a dense core much smaller than the atom.
Can neutrons be manipulated?
3. neutrons have no charge and thus could not be manipulated in electric or magnetic fields.
How many neutrons are in magnesium?
Magnesium has an atomic mass of 24.3. there are two isotopes of magnesium - one contains 12 neutrons and the other contains 13 neutrons in the nucleus. what is the fractional abundance of the one that contains 12 neutrons?
What is the atomic number of magnesium?
Magnesium's atomic number is 12 , so the natural occurring isotope for magnesium is Mg-12 (12 protons and 12 neutrons). Added up we have an atomic mass of 24 amu. Which means if we added one neutron in Mg-13, our atomic mass would be 25 amu.
What is the fractional abundance of Mg-12?
So in this case, the fractional abundance of Mg-12 would be .7, or 70%.