Nitrogen forms both covalent and ionic bonds. Nitrogen is a nonmetal with 5 electrons in its outer shell. Can Silicon form covalent bond with nitrogen? Nitrogen is a non metal. The electronegativity difference between silicon and non-metals such as chlorine is not very high. Because of this, silicon generally forms covalent compounds.
What are 5 examples of covalent bonds?
What are 5 examples of covalent bonds? Hydrogen (H 2) Hydrogen (H) is the simplest of all elements. … Oxygen (O 2) The valency of oxygen (O) is two, which means that it requires two electrons to complete its outermost (valence) shell. … Nitrogen (N 2) … Water (H 2 O) … Carbon Dioxide (CO 2) … Methane (CH 4) … Ammonia (NH 3) …
How many atoms are in a covalent bond?
The covalent bonds include pairs of electrons by two atoms binding them in a fixed orientation. While a bond between two ions is called ionic bonds.
What are the names of covalent compounds?
We have already encountered these compounds, but we list them here explicitly:
- H 2 O: water
- NH 3: ammonia
- CH 4: methane
What is an example of a covalent bond?
There are the following types of covalent bond:
- Simple . Atoms share a pair of electrons from their last shell (one electron from each). ...
- Double . Atoms contribute two electrons each, forming a four-electron double bond. ...
- Triple . Atoms contribute three electrons to form three electronic pairs, that is, six electrons in total forming the triple bond. ...
- Dative . ...
What elements can bond with nitrogen?
Chemical properties At room temperature, nitrogen is a very inactive gas. It does not combine with oxygen, hydrogen, or most other elements. Nitrogen will combine with oxygen, however, in the presence of lightning or a spark.
Can Silicon form a covalent bond with nitrogen?
Silicon can form stable covalent bonds with the same crucial elemental building blocks as carbon. It can covalently bind itself, carbon, nitrogen, oxygen, sulfur, phosphorus, and the halogens, as well as semi-metals like germanium. Silicon can also form covalent bonds with many metals [31,32].
Which elements can form covalent bonds?
Covalent bonds usually occur between nonmetals. For example, in water (H2O) each hydrogen (H) and oxygen (O) share a pair of electrons to make a molecule of two hydrogen atoms single bonded to a single oxygen atom. In general, ionic bonds occur between elements that are far apart on the periodic table.
Which bond is formed in nitrogen?
A covalent bond is formed between two nitrogen atoms.
Why does nitrogen only form covalent bonds?
Two covalent bonds form between the two oxygen atoms because oxygen requires two shared electrons to fill its outermost shell. Nitrogen atoms will form three covalent bonds (also called triple covalent) between two atoms of nitrogen because each nitrogen atom needs three electrons to fill its outermost shell.
Does nitrogen form only covalent?
The hydrogen atom and the halogen atoms form only one covalent bond to other atoms in stable neutral compounds. However, the carbon, oxygen, and nitrogen atoms can bond to more than one atom....Covalent Bonds.AtomValenceNitrogen3Carbon47 more rows
What are 5 examples of covalent bonds?
Examples of Covalent BondsHydrogen (H2) Hydrogen (H) is the simplest of all elements. ... Oxygen (O2) The valency of oxygen (O) is two, which means that it requires two electrons to complete its outermost (valence) shell. ... Nitrogen (N2) ... Water (H2O) ... Carbon Dioxide (CO2) ... Methane (CH4) ... Ammonia (NH3) ... Carbon Monoxide (CO)
How many bonds can nitrogen form?
3 bondsNitrogen will usually have 3 bonds, occasionally 4; however, if the N has 4 bonds it will be positively charged. Nitrogen can also have 2 bonds if the nitrogen atom is negatively charged. Oxygen will usually have 2 bonds, occasionally 3; however, if the O has 3 bonds it will be positively charged.
How do you find a covalent bond?
of covalent bond of a compound -short trick. Hello, The number of bonds for neutral atom is equal to the number of electrons in the full valence shell (2 or 8 electrons) minus the number of valence electrons.
How can nitrogen make 4 bonds?
Nitrogen atoms donate four electrons to form N4+ ions and oxygen atoms gain two electrons to form O2− ions when nitrogen and oxygen form an ionic bond.
What type of bond is nh3?
covalent bondsAmmonia contains nitrogen and hydrogen, which are both nonmetals. So nitrogen forms three covalent bonds with the three hydrogen present.
Is O2 a covalent bond?
Hence, O 2 is a covalent bond.
Can silicon form covalent bonds?
Silicon (Si) is tetravalent in nature just like Carbon (C) . That means it can easily share all four of its valence electrons to form covalent bonds with other atoms or molecules. So in order to be stable, Silicon(Si)needs to form four covalent bonds. Like Carbon (C), it can form covalent bonds with Hydrocarbons.
What type of bonds does silicon make?
Silicon atom forms four covalent bonds with the four neighboring atoms. In covalent bonding each valence electron is shared by two atoms.
Which type of bond is formed by silicon?
covalent bondingThe semiconductors such as silicon and germenium contains covalent bonding. Two adjacent atoms share electron and form a covalent bond.
Does Si have covalent bonding?
Silicon crystallises in a giant covalent structure at standard conditions, specifically in a diamond cubic lattice. It thus has a high melting point of 1414 °C, as a lot of energy is required to break the strong covalent bonds and melt the solid.
Which two elements are most likely to form covalent bonds with each other?
3) Based on their location in the periodic table, nitrogen (N) and oxygen (O) are most likely to form covalent bonds with each other
Which element will most likely lose electrons to form positive ions when bonding with other elements?
Therefore, the element that will most likely lose electrons to form positive ions when bonding with other elements is rubidium (Rb).
What is electronegativity in chemistry?
According to Linus Pauling, electronegativity refers to the ability of an element in a compound to draw electrons towards itself.
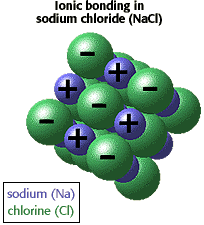
Which atom transfers electrons to the chlorine atoms to form ionic bonds?
The sodium atom transfers electrons to the chlorine atoms to form ionic bonds.
How is sodium chloride formed?
Sodium chloride is an ionic compound. It is formed by transfer of electrons from sodium to chlorine. Sodium is highly electropositive while chlorine is highly electronegative. Therefore, sodium chloride is formed when sodium atom transfers electrons to the chlorine atoms to form ionic bonds.
Why do atoms form chemical bonds?from quizlet.com
Atoms form chemical bonds because they want a lower energy and more stable electron configuration. (p. 149)
What is chemical bonding?from quizlet.com
A chemical bond that involves sharing a pair of electrons between atoms in a molecule.
How many valance electrons does helium have?from quizlet.com
The first four or five elements usually bond to attain the electron configuration of helium, which has only two valance electrons, and transitions often share more than their eight valance electrons. (p. 149)
How many electrons does hydrogen need?from quizlet.com
Hydrogen needs only one electron to complete its balance electron shell and is a diatomic element, similar to a group 17. (pp. 153-154)
