Things to Remember
- Aldehydes and ketones are organic molecules that contain the C=O carbonyl functional group. ...
- An aldehyde is an organic chemical with the structure C (H)=O that contains a functional group. ...
- A ketone is a functional group in chemistry having the formula R?C=O, where R can be any carbon-containing substituent. ...
What does aldehyde group stand for?
aldehyde, any of a class of organic compounds in which a carbon atom shares a double bond with an oxygen atom, a single bond with a hydrogen atom, and a single bond with another atom or group of atoms (designated R in general chemical formulas and structure diagrams). The double bond between carbon and oxygen is characteristic of all aldehydes and is known as the carbonyl group.
What functional group is common to all alcohols?
Alcohols contain the hydroxy functional group (-OH), bonded to a carbon atom of an alkyl or substituted alkyl group. The functional group of an alcohol is the hydroxyl group, –OH.
What are the properties of functional groups?
Properties of Functional Groups. A functional group can participate in specific chemical reactions. Some of the important functional groups in biological molecules include: hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl groups. These groups play an important role in the formation of molecules like DNA, proteins, carbohydrates, and lipids.
What is the difference between aldehydes and ketones?
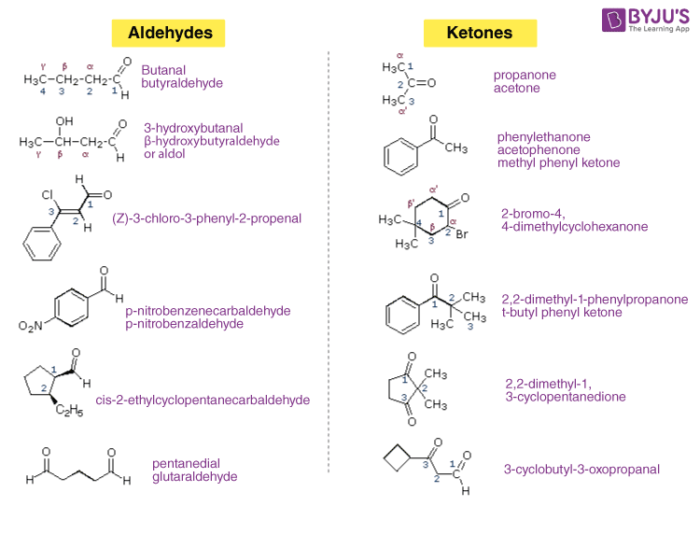
They both have a carbonyl functional group, but the main difference is in the atoms bonded to the central carbon. While aldehydes have an R group chain of hydrocarbon substituents and a hydrogen attached to the central carbon, ketones have R and R’ substituents attached to the central carbon.
Is functional group in aldehyde *?
Aldehydes and ketones are organic compounds that have a carbonyl functional group.
Which functional group is found in aldehydes quizlet?
The functional group aldehydes is the C=O. (carbonyl) group. In formaldehyde, CH2O, the simplest aldehyde, the carbonyl carbon is bonded to two hydrogens. In all other aldehydes, it is bonded to one hydrogen and one carbon.
Which functional group found is found in a carboxylic acid?
carboxyl functional groupA carboxylic acid is an organic compound that contains the carboxyl functional group. The general formula for a carboxylic acid can be abbreviated as R−COOH. The carbon atom of the carboxyl group may be attached to a hydrogen atom or to a carbon chain.
Which functional group is found in an amine?
The functional group of an amine is a nitrogen atom with a lone pair of electrons and with one, two, or three alkyl or aryl groups attached. The amide. functional group has a carbonyl group joined to a nitrogen atom from ammonia or an amine.
What is an aldehyde quizlet?
Aldehyde. the carbon of the carbonyl group is bonded to at least one hydrogen atom and may also be bonded to another hydrogen atom, a carbon of an alkyl group or an aromatic ring. Ketone.
Which of the following is an example of an aldehyde?
1 Answer. Formaldehyde, Acetaldehyde, Benzaldehyde, Citral.
How are aldehydes different from ketones quizlet?
Because aldehydes always contain at least one hydrogen bonded to the C=O. group, they are often written RCH=O. The functional group of a ketone is a carbonyl group bonded to two carbon atoms.
Which of the following best describes the term aldehyde?
Which of the following best describes the term aldehyde? An aldehyde is an organic compound containing a carbonyl group attached to a hydrogen.
What is the chemical structure of aldehyde?
Chemically, an aldehyde / ˈældɪhaɪd / is a compound containing a functional group with the structure −CHO, consisting of a carbonyl center (a carbon double-bonded to oxygen) with the carbon atom also bonded to hydrogen and to any generic alkyl or side chain R group.
What are the two aldehydes that are important?
The two aldehydes of greatest importance in industry, formaldehyde and acetaldehyde, have complicated behavior because of their tendency to oligomerize or polymerize. Formaldehyde in particular is sold as the polymer paraformaldehyde ( (C2H5O) n, typically n = 50 to 100) as well as the trimer 1,3,5-trioxane (metaformaldehyde). In addition to the inconveniently low-boiling monomer (b.p. 21 °C), acetalde hyde is available as the trimer paraldehyde (a sedative and anticonvulsant) and tetramer metaldehyde (a slug and snail poison). In general, higher aliphatic aldehydes will accumulate a substantial amount of oligomer (mostly trimer) upon long-term storage and must be freshly distilled when a reaction calls for the monomeric starting material. They also tend to hydrate, forming the geminal diol. Formaldehyde is often sold as the aqueous solution, formalin, which is mostly 1,1-methanediol, with a small amount of methanol added as stabilizer. The oligomers/polymers and the hydrates exist in equilibrium with the parent aldehyde, and for some synthetic procedures, they can serve as substitutes for the anhydrous monomer.
What is the center of an aldehyde?
Aldehydes feature an sp 2 - hybridized, planar carbon center that is connected by a double bond to oxygen and a single bond to hydrogen. The C–H bond is not ordinarily acidic. Because of resonance stabilization of the conjugate base, an α-hydrogen in an aldehyde (not shown in the picture above) is far more acidic, with a p Ka near 17, compared to the acidity of a typical alkane (p Ka about 50). This acidification is attributed to (i) the electron-withdrawing quality of the formyl center and (ii) the fact that the conjugate base, an enolate anion, delocalizes its negative charge. Related to (i), the aldehyde group is somewhat polar. The formyl proton itself does not readily undergo deprotonation. The anionic species formally derived from deprotonation of an aldehyde proton, known as an acyl anion, is highly unstable and must be kept at low temperatures. In fact, with the exception of certain hindered dialkylformamides, the synthesis of acyl anions by direct deprotonation is not a feasible route, since the deprotonated species will almost immediately add to the highly reactive carbonyl of the starting material to form an acyloin compound. For this reason, the acidity of the formyl proton is difficult to measure. In the case of HCON i Pr 2, the acidity of the formyl group was found to be very close to that of diisopropylamine (p Ka ~ 36). The gas-phase acidity of aldehyde was found to be 1,640 kJ/mol (393 kcal/mol), making it more acidic than hydrogen (1,700 kJ/mol, 400 kcal/mol) and ammonia (1,680 kJ/mol, 402 kcal/mol), but less acidic than water (1,600 kJ/mol, 390 kcal/mol) in the gas phase.
Why are aldehydes not common in building blocks?
Possibly because of the high reactivity of the formyl group, aldehydes are not common in several of the natural building blocks: amino acids, nucleic acids, lipids. Most sugars, however, are derivatives of aldehydes. These aldoses exist as hemiacetals, a sort of masked form of the parent aldehyde.
What is the functional group of an alcohol?
The functional group itself (i.e. without the "R" side chain) is known as an aldehyde or formyl group . Aldehydes, which are generally created by removing a hydrogen from an alcohol, are common in organic chemistry; the most well-known is formaldehyde.
What is the name of the acyclic aliphatic aldehyde?
Acyclic aliphatic aldehydes are named as derivatives of the longest carbon chain containing the aldehyde group. Thus, HCHO is named as a derivative of methane, and CH 3 CH 2 CH 2 CHO is named as a derivative of butane.
How are formaldehydes made?
Aldehydes are commonly generated by alcohol oxidation. In industry, formaldehyde is produced on a large scale by oxidation of methanol. Oxygen is the reagent of choice, being "green" and cheap. In the laboratory, more specialized oxidizing agents are used, but chromium (VI) reagents are popular. Oxidation can be achieved by heating the alcohol with an acidified solution of potassium dichromate. In this case, excess dichromate will further oxidize the aldehyde to a carboxylic acid, so either the aldehyde is distilled out as it forms (if volatile) or milder reagents such as PCC are used.
How are aldehydes and ketones related?
Ketones and aldehydes are two closely related carbonyl-based functional groups that react in very similar ways. In a ketone, the carbon atom of a carbonyl is bonded to two other carbons. In an aldehyde, the carbonyl carbon is bonded on one side to a hydrogen, and on the other side to a carbon.
What are functional groups?
Functional groups are structural units within organic compounds that are defined by specific bonding arrangements between specific atoms. For example the structure of capsaicin, found in chili peppers, incorporates several functional groups, labeled in the figure below and explained throughout this section.
What are alkenes and alkynes?
Alkenes (sometimes called olefins) have carbon-carbon double bonds, and alkynes have carbon-carbon triple bonds. Ethene, the simplest alkene example, is a gas that serves as a cellular signal in fruits to stimulate ripening. (If you want bananas to ripen quickly, put them in a paper bag along with an apple – the apple emits ethene gas, setting off the ripening process in the bananas). Ethyne, commonly called acetylene, is used as a fuel in welding blow torches.
What is the name of the group of alkyl halides?
When the carbon of an alkane is bonded to one or more halogens, the group is referred to as a alkyl halide or haloalkane. Chloroform is a useful solvent in the laboratory, and was one of the earlier anesthetic drugs used in surgery. Chlorodifluoromethane was used as a refrigerant and in aerosol sprays until the late twentieth century, but its use was discontinued after it was found to have harmful effects on the ozone layer. Bromoethane is a simple alkyl halide often used in organic synthesis. Alkyl halides groups are quite rare in biomolecules.
What is the aromatic group?
The aromatic group is exemplified by benzene (which used to be a commonly used solvent in the organic lab, but which was shown to be carcinogenic), and naphthalene, a compound with a distinctive ‘ mothball’ smell. Aromatic groups are planar (flat) ring structures, and are widespread in nature.
What is the default in organic chemistry?
The ‘default’ in organic chemistry (essentially, the lack of any functional groups ) is given the term alkane, characterized by single bonds between carbon and carbon, or between carbon and hydrogen. Methane, CH4, is the natural gas you may burn in your furnace. Octane, C8H18, is a component of gasoline.
Why is it important to recognize functional groups?
As we progress in our study of organic chemistry, it will become extremely important to be able to quickly recognize the most common functional groups, because they are the key structural elements that define how organic molecules react. For now, we will only worry about drawing and recognizing each functional group, as depicted by Lewis and line structures. Much of the remainder of your study of organic chemistry will be taken up with learning about how the different functional groups behave in organic reactions.
CORE Concepts
Topics Covered in Other Articles
What Is An aldehyde?
- An aldehyde is a common functional group in organic chemistry. In addition, they are primarily derivatives of alcohols. Aldehydes are responsible for natural and synthetic hormones. The structure of an aldehyde consists of a carbonyl groupsingle bonded to a hydrogen atom. The carbonyl carbon is double bonded to an oxygen atom, and it is bonded to a...
Naming Aldehydes
- For naming aldehydes, the IUPAC nomenclature is more precise than using common naming. In IUPAC naming, the “-e” suffix is removed from the parent alkane chains and replaced with “-al”. However, if the aldehyde is attached to a ring, the “-carbaldehyde” suffix is added to the parent alkane name instead. The functional group of an aldehyde is always located at the lowest possi…
Reactions of Aldehydes
- Aldehydes are a fairly reactive functional group. This is due to the carbon of the aldehyde which contains a partial positive charge. Overall, aldehydes can undergo many addition reactions such as reacting with Grignard reagents. Some reactions that aldehydes can undergo are the following: Nucleophilic addition: Reacting with Grignard reagents: Reduction with NaBH4 or LiAlH4: Hydrati…
Real Life Examples of Aldehydes
- Aldehydes are in our everyday lives. Down below are two examples of compounds with aldehyde groups and their functions.
Further Reading
Overview
In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres.
Structure and bonding
Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp -hybridized. The aldehyde group is somewhat polar. The C=O bond length is about 120-122 picometers.
Physical properties and characterization
Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile aldehydes have pungent odors.
Aldehydes can be identified by spectroscopic methods. Using IR spectroscopy, they display a strong νCO band near 1700 cm . In their H NMR spectra, the formyl hydrogen center absorbs ne…
Applications and occurrence
Important aldehydes and related compounds. The aldehyde group (or formyl group) is colored red. From the left: (1) formaldehyde and (2) its trimer 1,3,5-trioxane, (3) acetaldehyde and (4) its enol vinyl alcohol, (5) glucose (pyranose form as α-D-glucopyranose), (6) the flavorant cinnamaldehyde, (7) the visual pigment retinal, and (8) the vitamin pyridoxal.
Synthesis
There are several methods for preparing aldehydes, but the dominant technology is hydroformylation. Illustrative is the generation of butyraldehyde by hydroformylation of propene:
H2 + CO + CH3CH=CH2 → CH3CH2CH2CHO
Aldehydes are commonly generated by alcohol oxidation. In industry, formaldehyde is produced on a large scale by oxidation of methanol. Oxygen is the reagent of choice, being "green" and che…
Common reactions
Aldehydes participate in many reactions. From the industrial perspective, important reactions are (a) condensations, e.g., to prepare plasticizers and polyols, and (b) reduction to produce alcohols, especially "oxo-alcohols". From the biological perspective, the key reactions involve addition of nucleophiles to the formyl carbon in the formation of imines (oxidative deamination) and hemiacetals (structures of aldose sugars).
Dialdehydes
A dialdehyde is an organic chemical compound with two aldehyde groups. The nomenclature of dialdehydes have the ending -dial or sometimes -dialdehyde. Short aliphatic dialdehydes are sometimes named after the diacid from which they can be derived. An example is butanedial, which is also called succinaldehyde (from succinic acid).
Biochemistry
Some aldehydes are substrates for aldehyde dehydrogenase enzymes which metabolize aldehydes in the body. There are toxicities associated with some aldehydes that are related to neurodegenerative disease, heart disease, and some types of cancer.