What are basic functional groups? Functional groups include: hydroxyl A hydroxy or hydroxyl group is the entity with the formula OH. It contains oxygen bonded to hydrogen. In organic chemistry, alcohol and carboxylic acids contain hydroxy groups. The anion, called hydroxide, consists of a hydroxyl group. In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl grou… A Phosphate is a chemical derivative of phosphoric acid. The phosphate ion³⁻ is an inorganic chemical, the conjugate base that can form many different salts. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Of the various phosphoric acid…Hydroxy group
Carbonyl group
Phosphate
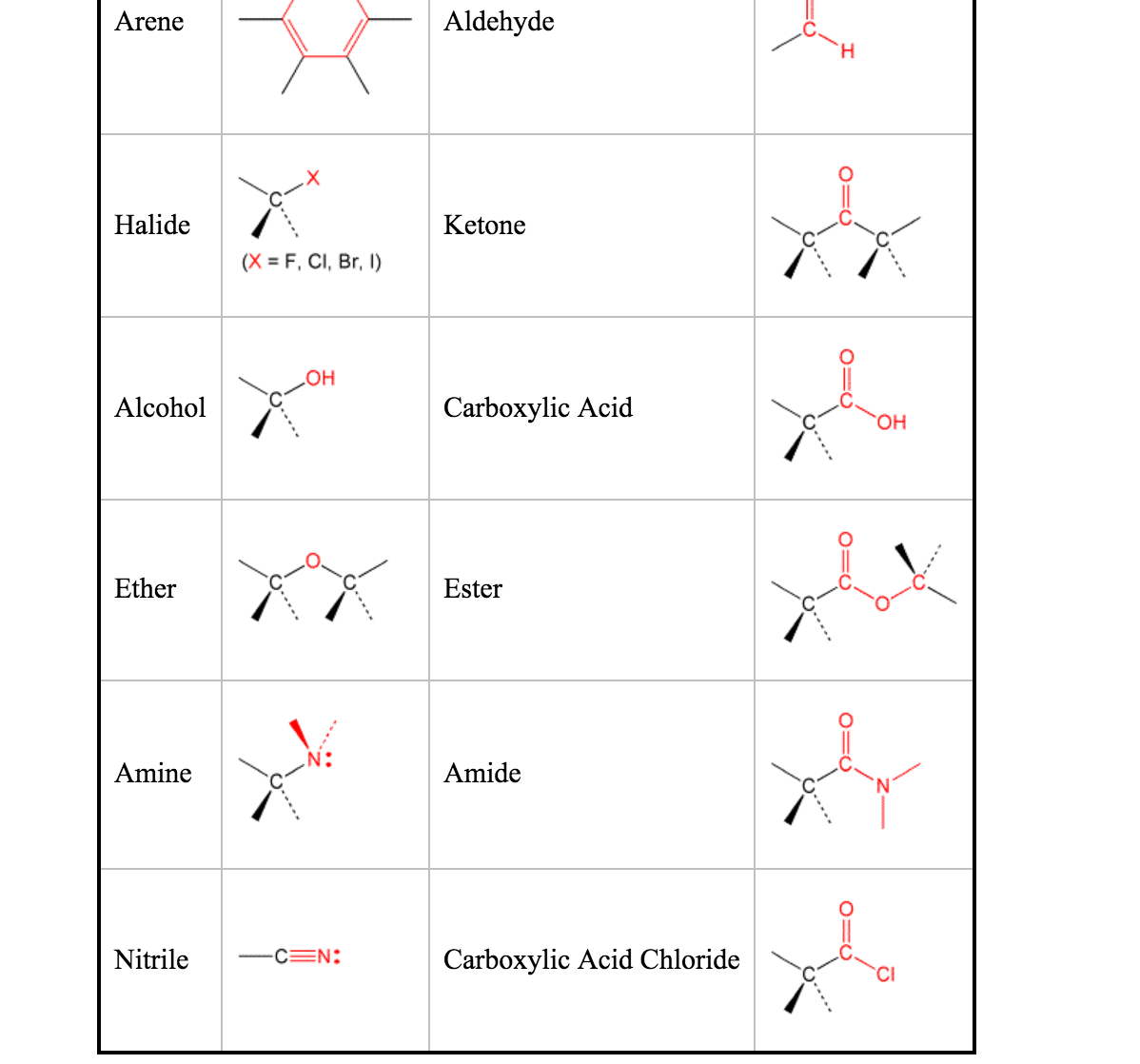
What are six examples of functional groups?
What are some examples of functional groups?
- Hydroxyl Functional Group.
- Aldehyde Functional Group.
- Ketone Functional Group.
- Amine Functional Group.
- Amino Functional Group.
- Amide Functional Group.
- Ether Functional Group.
- Ester Functional Group.
What are functional groups and their properties?
Functional Groups and Their Properties. What is a functional group? • A structural arrangement of atoms which determines the physical and chemical properties in a given molecule • Organic molecules are grouped into organic families based on the functional groups present
What do all functional groups have in common?
functional groups is used to describe the pieces or parts of a drug molecule. The key point here is that each individual group within a drug molecule can serve to provide one or more specifi c roles, tasks, or functions. As evidenced by functional groups A and B, the same functional group—a carboxylic acid in this case—can serve different roles
What functional groups are found in all?
There are 7 important functional groups in the chemistry of life: Hydroxyl, Carbonyl, Carboxyl, Amino, Thiol, Phosphate, and aldehyde groups.
Which functional groups are bases?
Amino groups can also act as bases, which means that the nitrogen atom can bond to a third hydrogen atom as shown in the image below. Once this occurs, the nitrogen atom gains a positive charge and can now participate in ionic bonds. The amine functional group can exist in a deprotonated or protonated state.
How do you know if a functional group is basic?
Since carboxyl groups can release H+ ions into a solution, they are considered acidic. Charged, accepts H+ to form NH3+. Since amino groups can remove H+ from solution, they are considered basic.
What is the most basic functional group?
In organic chemistry, the most common functional groups are carbonyls (C=O. ... Alcohols are functional groups characterized by the presence of an -OH group.Alcohols are organic compounds in which the hydroxyl functional group (-OH ) is bound to a carbon atom.More items...
Are functional groups acidic or basic?
The functional groups are mainly classified into acidic and basic functional groups. Representative surface acidic functional groups are carbonyl, carboxyl, phenolic hydroxyl, and lactone groups.
Is a hydroxyl group acidic or basic?
acidicH 3O + is called a hydronium ion, and it makes things acidic. OH - is called a hydroxyl ion and it makes things basic.
Are carboxyl groups acidic or basic?
The chief chemical characteristic of the carboxylic acids is their acidity. They are generally more acidic than other organic compounds containing hydroxyl groups but are generally weaker than the familiar mineral acids (e.g., hydrochloric acid, HCl, sulfuric acid, H2SO4, etc.). Carboxylic acids occur widely in nature.
How do you tell which is more basic?
the more stable a lone pair of electrons is, the less basic it will be. the less stable a lone pair of electrons is, the more basic it will be.
Which of the following is most basic?
Basicity is enhanced by the presence of electron donating group & availability of a lone pair of electrons on the nitrogen atom. Hence is most basic.
How do you know which compound is most basic?
First, scan the molecule for all non-halogen atoms with lone pairs (usually N and O). Second, imagine protonating each candidate atom and draw its conjugate acid. Third, identify the weakest conjugate acid. The protonated atom in the weakest conjugate acid is the most basic atom in the original molecule.
Is amine acidic or basic?
basicAmine are basic and easily react with the hydrogen of acids which are electron poor as seen below. Amines are one of the only neutral functional groups which are considered basis which is a consequence of the presence of the lone pair electrons on the nitrogen.
Are aldehydes acidic?
The α- hydrogen atoms of aldehydes and ketones are acidic in nature.
What functional groups are neutral?
Neutral compounds include the following functional group classes: hydrocarbons (alkanes, alkenes, alkynes, aromatic compounds), alcohols, aldehydes, ketones, esters, amides, nitro compounds.
How do you identify functional groups?
Identification and extraction of functional groupsmark all heteroatoms in a molecule, including halogens.mark also the following carbon atoms: atoms connected by non-aromatic double or triple bond to any heteroatom. atoms in nonaromatic carbon–carbon double or triple bonds. ... merge all connected marked atoms to a single FG.
How do you know if A functional group is polar or nonpolar?
When 2 equally strong (electronegative) atoms are bound, the sharing of electrons will be equal between them. If a functional group is composed of an atom that has strong-weak bonds, the group will be polar.
Are amides basic or acidic?
Compared to amines, amides are very weak bases. While the conjugate acid of an amine has a pKa of about 9.5, the conjugate acid of an amide has a pKa around −0.5. Therefore, amides don't have as clearly noticeable acid–base properties in water.
What functional group identifies organic acids?
carboxyl groupThe organic acids are known as carboxylic acid as the functional group is carboxyl group. This is the structure of the organic acid where R can be an alkyl or benzyl group and –COOH is the functional group.
What are functional groups?
Functional groups are groups of atoms that occur within organic molecules and confer specific chemical properties to those molecules. When functional groups are shown, the organic molecule is sometimes denoted as “R.”.
What are functional groups in macromolecules?
Functional groups are found along the “carbon backbone” of macromolecules which is formed by chains and/or rings of carbon atoms with the occasional substitution of an element such as nitrogen or oxygen. Molecules with other elements in their carbon backbone are substituted hydrocarbons. Each of the four types of macromolecules—proteins, lipids, carbohydrates, and nucleic acids—has its own characteristic set of functional groups that contributes greatly to its differing chemical properties and its function in living organisms.
How many bonds does carbon have?
Carbon has four electrons in its outermost shell and can form four bonds. Carbon and hydrogen can form hydrocarbon chains or rings. Functional groups are groups of atoms that confer specific properties to hydrocarbon ...
Which functional group is hydrophilic?
Among the hydrophilic functional groups is the carboxyl group found in amino acids, some amino acid side chains, and the fatty acid heads that form triglycerides and phospholipids. This carboxyl group ionizes to release hydrogen ions (H +) from the —COOH group resulting in the negatively charged —COO – group; this contributes to ...
Which functional group has a partially negatively charged oxygen atom that may form hydrogen bonds with water molecules?
Other functional groups, such as the carbonyl group, have a partially negatively charged oxygen atom that may form hydrogen bonds with water molecules, again making the molecule more hydrophilic. Table 1. Important Functional Groups in Biology. Charged, ionized to release H +.
Which macromolecules have their own functional groups?
Each of the four types of macromolecules—proteins, lipids, carbohydrates, and nucleic acids —has its own characteristic set of functional groups that contributes greatly to its differing chemical properties and its function in living organisms.
What are hydrogen bonds?
Hydrogen bonds are also involved in various recognition processes, such as DNA complementary base pairing and the binding of an enzyme to its substrate, as illustrated in Figure 1. Figure 1. Hydrogen bonds connect two strands of DNA together to create the double-helix structure.
What are functional groups in chemistry?
Functional groups are collections of atoms in organic chemistry molecules that contribute to the chemical characteristics of the molecule and participate in predictable reactions. These groups of atoms contain oxygen or nitrogen or sometimes sulfur attached to a hydrocarbon skeleton. Organic chemists can tell a lot about a molecule by ...
What is functional group?
Key Takeaways: Functional Groups. In organic chemistry, a functional group is a set of atoms within molecules that function together to react in predictable ways. Functional groups undergo the same chemical reactions no matter how large or small the molecule is. Covalent bonds link the atoms within functional groups and connect them to the rest ...
What is the structure of a hydroxyl group?
Also known as the alcohol group or hydroxy group, the hydroxyl group is an oxygen atom bonded to a hydrogen atom. Hydroxy groups link biological molecules together via dehydration reactions.
What is an ether group?
An ether group consists of an oxygen atom forming a bridge between two different parts of a molecule.
How can organic chemists tell a lot about a molecule?
Organic chemists can tell a lot about a molecule by the functional groups that make up a molecule. Any serious student should memorize as many as they can. This short list contains many of the most common organic functional groups. It should be noted that the R in each structure is a wildcard notation for the rest of the molecule's atoms.
What are some examples of compounds that contain hydroxyl groups?
Examples of common compounds containing hydroxyl groups are alcohols and carboxylic acids.
What is the carboxyl group?
The carboxyl group is an ester where one substituent R is a hydrogen atom.
