
Which has the highest boiling point H2S or H2Te?
In this case, we have to look at the molecular weight. H2Te has the highest molecular weight and thus it has the highest boiling point from H2S and H2Se. Why does H2SE have a higher boiling point than H2S?
Why does hydrogen sulfide have a lower boiling point than water?
Also, hydrogen sulfide has weaker intermolecular forces (dipole forces, vander waal’s forces) compared to water resulting in a lower melting point and boiling point. Why is the boiling point of H2S higher than HCl despite HCl being more polar than H2S?
What is the difference between H2O and H2S?
In H2O, H2 is bind with oxygen which Is more electro negative therefore polarity develops on H2O…..and hydrogen bonding occur between the water molecule…..Since hydrogen bonding is very strong..so large amt of energy is required to break this bond…… On the other hand, in h2S...
What is the stronger intermolecular bonding between H2O and HTe?
Between H2O and HTe, what is the stronger intermolecular bonding, and why? Water exhibits hydrogen bonding and all three van der Waals forces: Keesom forces (dipole-dipole attraction), Debye forces (induced attraction) and London dispersion forces (which all molecules exhibit).
Why is H2O have a higher boiling point than H2S?
Between water and hydrogen sulfide, both are polar, and have dipole-dipole forces, so they have higher boiling points than methane or silane. But water has hydrogen bonds, which are extra-strong dipole-dipole forces. Water boils much hotter than hydrogen sulfide.
Why H2S has lower boiling point than H2O?
H2S molecules are involved in weaker hydrogen bonding than H2O molecules. This is because of the lower electronegativity of sulphur than oxygen. So, less energy is required to break the hydrogen bonds in H2S. Thus, the boiling point of H2S is lower than that of H2O.
Which one has the lowest boiling point H2O H2S?
Explanation. H2O > H2Te > H2Se > H2S. Hence H2S has the lowest boiling point.
Which has a higher surface tension H2O or H2S?
d) Water has a higher surface tension compared to H2S. e) Water has a higher viscosity compared to H2S. 15. Ethyl ether molecules experience only London dispersion forces whereas hydrogen sulfide (H2S) molecules experience dipole forces.
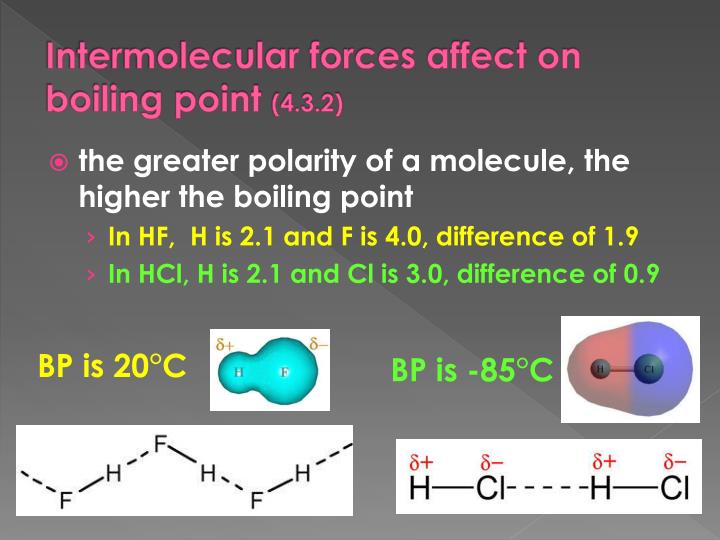
Why does H2O have a higher boiling point than HF?
By the way we all know, why H2O,NH3,HF has significantly higher boiling point than the other period members, yes, because of HYDROGEN BONDING. It is obvious that, more the electronegative element the H attached to, the more will be its magnitude of H Bond. So it the hydrogen-bond strength follows order HF> H2O>NH3. Still, H2O has higher boiling point than HF, the reason is as follows.
Which element has the highest boiling point?
For the given hydrides of group 16 elements, H2O has hydrogen bonding between the its molecule and rest of the compounds have vander waals interaction. Hydrogen bonding is stronger interaction, so H2O has highest boiling point. Other compounds have vander waals interaction, so now we have to consider the magnitude of interaction. Vander waals interacti
Why is water more polar than hydrogen sulfide?
Water is more polar than hydrogen sulfide because the oxygen atom is more electronegative than the sulfur atom. Also, hydrogen sulfide has weaker intermolecular forces (dipole forces, vander waal’s forces) compared to water resulting in a lower melting point and boiling point.
How many hydrogen bonds can a molecule of HF form?
HF is a linear molecule, a molecule of HF can only form 2 hydrogen bonds (as ---H-F---H-F---H-F---). But the shape of water molecule is 'V Shaped' due to the presence of 2 lone pairs (of course F in HF also has lone pairs, but it cant utilize it for H-bond due to its linear shape), and a molecule of H2O can form 4 hydrogen bonds (where HF there is only 2). Thus eventhough bond strength is high in HF, the combined bond strengths of 4 Hydrogen Bonds in H2O will overcome the combined bond strength of 2 Hydrogen bonds in HF. Thus H2O has more boiling point than HF...
How do hydrogen bonds work?
The four spare electrons give the central atom in the molecule a slight negative charge, and the hydrogen atoms become slightly positively charged. The negatively charged central atoms attract the positively charged hydrogen atoms from other molecules in a process known as hydrogen bonding. If each molecule does this, then eventually you'll have a rigid latticework of interconnected molecules (i.e. a solid). Give the solid enough energy and enough of those hydrogen bonds will break to form a liquid and (eventually) a gas.
Why is hydrogen sulfide weaker than water?
In short, because the intermolecular forces holding the molecules together in a rigid, solid structure are much weaker in hydrogen sulfide than in water. The weaker the forces, the less energy is required to break them and the lower the melting point.
How to predict boiling point?
Boiling point can be predicted on the basis of the interaction present between the molecules (more the interactions, more will be the boiling point). If same type of interaction is present, then we have to follow the magnitude of the interaction.
