
What is the scientific name for mica?
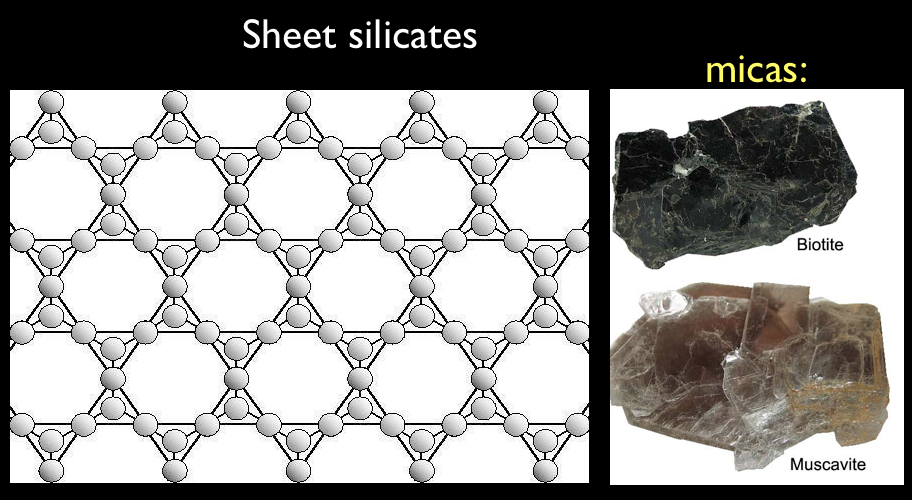
Minerals Database. Mica. Mica is a mineral name given to a group of minerals that are physically and chemically similar. They are all silicate minerals, known as sheet silicates because they form in distinct layers.
What are the characteristics of mica?
Description. Mica is a mineral name given to a group of minerals that are physically and chemically similar. They are all silicate minerals, known as sheet silicates because they form in distinct layers. Micas are fairly light and relatively soft, and the sheets and flakes of mica are flexible. Mica is heat-resistant and does not conduct ...
What is the mica group?
Statistics and information on the worldwide supply of, demand for, and flow of the mineral commodity mica The mica group represents 37 phyllosilicate minerals that have a layered or platy texture. The commercially important micas are muscovite and phlogopite.
What is a mica sheet?
Mica is a mineral name given to a group of minerals that are physically and chemically similar. They are all silicate minerals, known as sheet silicates because they form in distinct layers. Micas are fairly light and relatively soft, and the sheets and flakes of mica are flexible.

What is mica made of?
Mica is a mineral name given to a group of minerals that are physically and chemically similar. They are all silicate minerals, known as sheet silicates because they form in distinct layers. Micas are fairly light and relatively soft, and the sheets and flakes of mica are flexible. Mica is heat-resistant and does not conduct electricity. There are 37 different mica minerals. The most common include: purple lepidolite, black biotite, brown phlogopite and clear muscovite.
How is sheet mica recovered?
Sheet Mica Mining: Sheet mica is recovered by either sinking a shaft along the strike and dip of a pegmatite or by open-pit surface mining of semi-hard pegmatite ore.
What is ground mica used for?
The principal use of ground mica is in gypsum wallboard joint compound, where it acts as a filler and extender, provides a smoother consistency, improves workability, and prevents cracking. In the paint industry, ground mica is used as a pigment extender that also facilitates suspension due to its light weight and platy morphology.
Where is mica mined?
Sheet mica is no longer mined in the U.S. because of the high cost of mining, the small market, and the high capital risk. Most sheet mica is mined in India, where labor costs are comparatively low.
Is mica a mineral?
Mica is a mineral name given to a group of minerals that are physically and chemically similar. They are all silicate minerals, known as sheet silicates because they form in distinct layers. Micas are fairly light and relatively soft, and the sheets and flakes of mica are flexible.
Is mining mica risky?
In either case, it is a very economically risky mining procedure because of the cost involved in locating the vein and the unpredictability of the quality and quantity of the mica that might be recovered once the vein is located and worked.
What are mica sheets made of?from geologyscience.com
Micas have sheet structures whose primary gadgets include two polymerized sheets of silica (SiO4) tetrahedrons. Two such sheets are juxtaposed with the vertices in their tetrahedrons pointing towards each different; the sheets are go-linked with cations—as an example, aluminum in muscovite—and hydroxyl pairs entire the coordination of those cations (see parent). Thus, the go-related double layer is certain firmly, has the bases of silica tetrahedrons on each of its outer aspects, and has a terrible charge. The fee is balanced by means of singly charged massive cations—for example, potassium in muscovite—that join the go-linked double layers to shape the complete shape. The variations among mica species rely upon differences within the X and Y cations.
What are the properties of mica?from geologyscience.com
Properties of Mica Group Minerals 1 The perfect cleavage into thin elastic sheets is probably the most widely recognized characteristic of the micas. 2 The luster of the micas is usually described as splendent, but some cleavage faces appear pearly. 3 Mohs hardness of the micas is approximately 2 1/2 on cleavage flakes and 4 across cleavage. 4 Specific gravity for the micas varies with composition. The overall range is from 2.76 for muscovite to 3.2 for iron-rich biotite.
How is sheet mica recovered?from mineralseducationcoalition.org
Sheet Mica Mining: Sheet mica is recovered by either sinking a shaft along the strike and dip of a pegmatite or by open-pit surface mining of semi-hard pegmatite ore.
What is muscovite used for?from geologyscience.com
Sheets of muscovite of particular thicknesses are applied in optical instruments. Ground mica is used in many approaches which includes a dusting medium to prevent, as an instance, asphalt tiles from sticking to each other and also as a filler, absorbent, and lubricant. It is likewise used inside the manufacture of wallpaper to provide it a glittery lustre. Lepidolite has been mined as an ore of lithium, with rubidium generally recovered as a by-product. It is used inside the manufacture of warmth-resistant glass. Glauconite-rich greensands have found use inside the United States as fertilizer—e.G., on the coastal undeniable of New Jersey—and a few glauconite has been employed as a water softener because it has a excessive base-change capability and has a tendency to regenerate instead hastily.
Why are micas called sheet silicates?from mineralseducationcoalition.org
They are all silicate minerals, known as sheet silicates because they form in distinct layers. Micas are fairly light and relatively soft, and the sheets and flakes of mica are flexible. Mica is heat-resistant and does not conduct electricity. There are 37 different mica minerals.
What is ground mica used for?from mineralseducationcoalition.org
The principal use of ground mica is in gypsum wallboard joint compound, where it acts as a filler and extender, provides a smoother consistency, improves workability, and prevents cracking. In the paint industry, ground mica is used as a pigment extender that also facilitates suspension due to its light weight and platy morphology.
How hard is mica?from geologyscience.com
Mohs hardness of the micas is approximately 2 1/2 on cleavage flakes and 4 across cleavage.
What is the layer of mica?
The 2:1 layer of micas is composed of an octahedral sheet between two sheets of tetrahedra, as depicted in Figure 7. Micas can be classified as dioctahedral or trioctahedral, depending on the types and locations of cations in the octahedral sheets ( Table 3 ). In trioctahedral micas such as biotite, all three octahedral positions are filled, whereas in dioctahedral micas such as muscovite, only two out of three octahedral cations are filled. Isomorphic substitution in the mica structure creates negative charge, which results in a strong coulombic attraction for charge-compensating interlayer cations such as K, which is not exchanged in the standard cation exchange-capacity determination. The negative layer charge of micas arises by some combination of three mechanisms: (1) substitution of R 3+ (primarily Al or Fe) for Si 4+ in tetrahedral positions, (2) substitution of R 2+ for R 3+ in octahedral positions, and (3) vacancies in octahedral positions. The resultant layer charge may originate entirely within the tetrahedral sheet, or may originate entirely within the octahedral sheet in some species, or may come partly from both sheets. The layer charge of micas is expressed on a formula unit basis ( Table 3 ).
What is the name of the mineral that is found in soils and sedimentary rocks?
In soils and sedimentary rocks, mica in the clay fraction is usually identified as illite. Illitic minerals have been called ‘hydrous mica,’ ‘clay mica,’ and ‘sericite.’. Illite is commonly found to be dioctahedral, but its chemical composition and layer charge differ from that of muscovite (Table 3).
What are micas in soil?
The micas in soils are mainly inherited by soils from soil parent materials. Micas serve as precursors for expansible 2:1 phyllosilicates, i.e., vermiculites and smectites, to which micas may be transformed by replacement of the interlayer cations (usually K+) by hydrated cations.
Why do micas have K+?
With the exception of paragonite, a Na-bearing mica, the other micas have K + in the interlayer positions to satisfy the negative charge resulting from isomorphous substitution. Thus, micas are major K-bearing minerals in soils and as they weather, the nonexchangeable K is released for plant uptake.
What percentage of mica based pores are nanometric?
Mica -based pores represent 0.6–7% of the nanoporous system, of which 92.31–100% of micaceous pores are nanometric. Micaceous nanopores are most abundant in the mica rich MRSL microfacies followed by the OM/ILS and OMRS microfacies. Typically characterized by an elongate, but constrained pore morphology, these pores demonstrate the potential to contribute to nanometric permeability, but their limitations in width will likely result in a complex mode of hydrocarbon migration, due to small pore throat diameters.
Which rock has more Fe than illite?
Compared with illite, the octahedral sheet of glauconite contains considerably more Fe. After feldspars and quartz, micas are the third most extensive group of minerals in granitic and sialic (acid) rocks in general but less extensive in most mafic rocks.
Which mica is the most abundant?
Muscovite is the most abundant dioctahedral primary mica. In muscovite, excess negative layer charge results from the substitution of Al for Si in one out of each of four Si 4+ in the tetrahedral sheet positions. Biotite is the most common trioctahedral mica.
What are micas and amphiboles?
Micas and amphiboles are hydrous minerals stabilized by dissolved water in the melt, and hence usually crystallize at depth. Biotite is common in plutonic and volcanic felsic rocks across the silica-saturation spectrum but is not typically found in peralkaline rhyolites. Muscovite is restricted to peraluminous granites; it has only been described from a bare handful of rhyolites. Amphiboles occur in all types except peraluminous rocks and have a wide range of compositions; alkali amphiboles such as arfvedsonite are prominent in nepheline syenites and peralkaline granites. Amphiboles and micas decompose if the partial pressure of water in the melt falls below a critical level; for amphibole in volcanic rocks, this has been experimentally calibrated and exploited as a geospeedometer to estimate rates of magma uprise and degassing (Rutherford and Hill, 1993 ); the thickness of the decomposed rim on the crystal is a function of time since the onset of degassing. Nonetheless, alkali amphiboles such as arfvedsonite [Na 3 (Mg,Fe) 4 (Al,Fe)Si 8 O 22 (OH,F) 2] have been reported from the groundmass of phonolites, and peralkaline trachytes and rhyolites (Parker, 2019); in these cases the mineral may be stabilized at low pressures by a high fluorine content.
What is the crystal shape of mica?
Mica crystals can grow to a large size (at least 3 m) and very often exhibit a pseudo-hexagonal crystal shape, both in hand specimen and thin section ( Figs. 3 A, B and 6 A ). The perfect basal cleavage is along the weakly bound sheets of X ions (K, Na, etc.) lying between the tetrahedral layers and this {001} cleavage is a useful characteristic for identification of the mica group. The different micas can exhibit a wide range of colors in hand specimen: muscovite is pale and silvery, biotite is dark brown or black, lithium micas may be pink and the chromium and vanadium micas can be bright shades of green.
What type of rock is mica found in?
Micas are generally more extensive in fine-grained sediments and sedimentary rocks (e.g., shales) than in coarse-textured sedimentary rocks (e.g., sandstones). Shales and slates are usually rich in the fine-grained, illitic-type micas. Illitic-type micas are also important minerals in limestones.
How to remove mica from a disc?
1. Glue a mica disk to a sample stage using epoxy and wait until it has dried (~ 1–2 h). 2. Press a Scotch tape to the surface of the mica disk and then smoothly remove the tape from the mica. The top layer of the mica will be removed with the tape, which can be checked by inspecting the surface of the removed tape. 3.
What are micas in soil?
The micas in soils are mainly inherited by soils from soil parent materials. Micas serve as precursors for expansible 2:1 phyllosilicates, i.e., vermiculites and smectites, to which micas may be transformed by replacement of the interlayer cations (usually K +) by hydrated cations.
Why do micas have K+?
With the exception of paragonite, a Na-bearing mica, the other micas have K + in the interlayer positions to satisfy the negative charge resulting from isomorphous substitution. Thus, micas are major K-bearing minerals in soils and as they weather, the nonexchangeable K is released for plant uptake.
Where does mica come from?
Mica in soils is usually inherited from the parent rock and is likely to occur in soils derived from various igneous and metamorphic rocks, as well as from sediments derived from them. Muscovite, biotite, and phlogopite are the three most common mica group minerals in rocks, and consequently in soils.
Overview
Micas are a group of silicate minerals whose outstanding physical characteristic is that individual mica crystals can easily be split into extremely thin elastic plates. This characteristic is described as perfect basal cleavage. Mica is common in igneous and metamorphic rock and is occasionally found as small flakes in sedimentary rock. It is particularly prominent in many granites, pegmat…
Properties and structure
The mica group is composed of 37 phyllosilicate minerals. All crystallize in the monoclinic system, with a tendency towards pseudohexagonal crystals, and are similar in structure but vary in chemical composition. Micas are translucent to opaque with a distinct vitreous or pearly luster, and different mica minerals display colors ranging from white to green or red to black. Deposits of mica tend to have a flaky or platy appearance.
Classification
Chemically, micas can be given the general formula
X2Y4–6Z8O20(OH, F)4,
in which
X is K, Na, or Ca or less commonly Ba, Rb, or Cs; Y is Al, Mg, or Fe or less commonly Mn, Cr, Ti, Li, etc.; Z is chiefly Si or Al, but also may include Fe or Ti.
Occurrence and production
Mica is widely distributed and occurs in igneous, metamorphic and sedimentary regimes. Large crystals of mica used for various applications are typically mined from granitic pegmatites.
The largest documented single crystal of mica (phlogopite) was found in Lacey Mine, Ontario, Canada; it measured 10 m × 4.3 m × 4.3 m (33 ft × 14 ft × 14 ft) and weighed about 330 tonnes (320 long tons; 360 short tons). Similar-sized crystals were also found in Karelia, Russia.
Uses
The commercially important micas are muscovite and phlogopite, which are used in a variety of applications.
Mica's value is based on its unique physical properties: the crystalline structure of mica forms layers that can be split or delaminated into thin sheets usually causing foliation in rocks. These sheets are chemically inert, dielectric, elastic, fl…
Etymology
The word mica is derived from the Latin word mica, meaning a crumb, and probably influenced by micare, to glitter.
Early history
Human use of mica dates back to prehistoric times. Mica was known to ancient Indian, Egyptian, Greek, Roman, and Chinese civilizations, as well as the Aztec civilization of the New World.
The earliest use of mica has been found in cave paintings created during the Upper Paleolithic period (40,000 BC to 10,000 BC). The first hues were red (iro…
Health impact
Mica dust in the workplace is regarded as a hazardous substance for respiratory exposure above certain concentrations.
The Occupational Safety and Health Administration (OSHA) has set the legal limit (permissible exposure limit) for mica exposure in the workplace as 20 million parts per cubic foot (706,720,000 parts per cubic meter) over an 8-hour workday. The National Institute for Occupational Safety an…