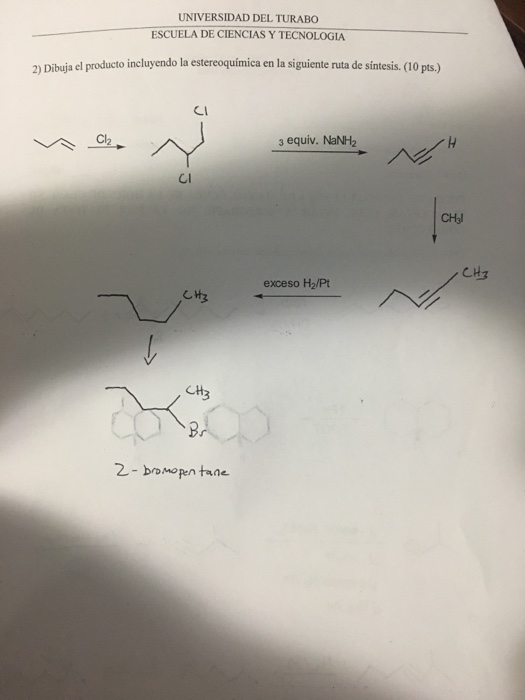
What is the difference between NaCl and AgCl?
NaCl is a ionic compound (polar nature)whereas AgCl is a covalent one (non polar). There is a rule in solution chemistry named " LIKE DISSOLVES LIKE".
Why is silver chloride more covalent than sodium chloride?
The bond character of Silver chloride is less ionic. The more ionic in character a bond is, the less covalent it will be. A covalent bond shares electrons, and an ionic bond does not share electrons. Thus, Silver chloride is more covalent because it shares its electrons more evenly than the sodium chloride does.
What is the covalency Order of LiCl and NaCl?
Similarly, NaCl is more covalent than Kcl. So, covalency order - LiCl>NaCl>KCL. For AgCl, Electronic configuration of Ag+ is - 4d 10 .
What is the percentage of ionic character in NaCl?
Therefore the percentage of ionic character of NaCl is 59.55%. What is the maximum ionic character in NaCl, CsCl, KCl, and RbCl?
See more

Is NaCl more ionic than AgCl?
Since the sodium is in period 3, and group 1a it is more electronegative than silver is. That means the bond character of Sodium Chloride will be more ionic. The bond character of Silver chloride is less ionic.
Why AgCl is less ionic than NaCl?
Solution : Ag' ion has high polarizing power due to pseudo inert gas configuration. `Na^(+)` has low polarizing so it poorly polarizes `Cl^(-)` ion. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams.
Is NaCl more covalent?
According to the statement above, in sodium chloride and sodium bromide, the cations are same, but the anions are different, as the size of the bromide ion is bigger than the size of the chloride ion, so it's polarisability will be higher than the chloride ion and thus sodium bromide is more covalent than sodium ...
Which of the following is most covalent CuCl NaCl AgCl?
Hence, AuCl is most covalent.
Why is AgCl most covalent?
Larger the cation size lesser the covalent character. Ag+ is larger than K+ so AgCl has less covalent character than KCl. Was this answer helpful?
Why does AgCl have a higher lattice energy than NaCl?
Because the bond in AgCl is ionic with partial covalent character, the lattice energy for AgCl is increased above that of NaCl. The greater cohesive forces in the AgCl crystal are largely responsible for the fact that AgCl has a very small solubility in water while NaCl is a soluble salt.
Which is more covalent CaCl2 or NaCl?
The larger the charge on cation, the greater is its polarising power, and more will be its covalent character. Since the charge on Calcium ion (+2) is greater than the charge on Sodium ion (+1), CaCl2 has a higher covalent character than NaCl.
Which has more covalent character NaCl or CaCl2?
Solution : (i) Due to higher charge , `Mg^(2)` is more polarising than `Na^(+)` and hence `MgCl_(2)` is more covalent than NaCl.
Why is NaCl not a covalent bond?
Covalent bonds occur when sharing of electrons exist between the atoms but in the case of the NaCl compound, the sodium atom completely transfers the electron to the chlorine atom, hence, there is no sharing between sodium and chlorine atom exist. Hence, the NaCl compounds can't be covalent in nature.
How do you find the most covalent?
The greater the electronegativity distinction, the more ionic a bond is. Electronegativity determines the bond with the most covalent character. Smaller electronegativity variations create a more covalent bond.
Which of the following is most covalent?
Among the given halides, AuCl is most covalent. This is because Au+ ion has psuedo noble gas configuration (18 electrons in the outermost shell). Hence, it has highest polarization power and is most covalent.
Which is more covalent bond?
∴ Among given choice C−S bond is most covalent in nature.
Why NaCl is soluble in water whereas AgCl is not?
Agcl is non -polar because of big size of silver ion with chlorine ion and so it's deform the electrons shells,so this gives its non polar nature so agcl insoluble in water ...
Why NaCl is more ionic than MgCl2?
In NaCl the net charge on each of the respective ions is + 1 whereas in MgCl2 the net charge is + 2, and the covalent characters directly depends upon the charges so lesser the charge lesser is the covalent character and more is ionic character .
Which is more ionic NaCl or KCl?
As we know larger the difference of electronegativity between atoms stronger (ionic)the bond . KCl is more ionic than NaCl.
Why is AgCl insoluble in water?
Complete answer: For $AgCl$ , $A{g^ + }$ has more polarizing power and thus as a result covalent character is developed in the compound. We can also say $AgCl$ has become non polar in nature. Since water is polar so it will dissolve only polar compounds . Hence $AgCl$ is insoluble in water.
Which is more covalent, sodium or potassium?
According to fajan's rule conditions-1) small size of cation 2)large size of anion, have more polarizability,which gives covalent character to any compound.And in this comparison,here size of anion is identical for both compounds (as they both contains Cl) ,so it all depends on size of cation,and on comparison we all know that sodium has small size as compared to potassium,so sodium chloride is more covalent than potassium chloride.
What is the difference between covalent and ionic bonds?
The more ionic in character a bond is, the less covalent it will be. A covalent bond shares electrons, and an ionic bond does not share e
What determines if a bond is covalent or ionic?
The more ionic in character a bond is, the less covalent it will be. A covalent bond shares electrons, and an ionic bond does not share e. Electronegativity in a bond determines the bond character as covalent or ionic. The more electronegative elements are furthest to the right in a period, and highest up in a family.
What are the factors that determine the melting point of an ionic compound?
Comparison of melting points of ionic compounds is generally done by considering the following two factors: Charge of the cation/anion : More the charge of cation or anion, stronger will be the forces of attraction between the ions and higher will be the melting point.
What is the electronic configuration of Ag+?
For AgCl, Electronic configuration of Ag+ is - 4d 10 . It has d block which is poorly screaning power than s,p-block which cannot effectively screaning the outermost electrons and they feel stronger attractive force towards nucleous and make it smaller in size than Li+,Na+,k+. Thus,Ag+ is more polarizable forms more covalency .
Which has a higher elctronegtivity, KCl or CaF?
KCl has higher elctronegtivity difference and the size of K is greater than Na atom (k is more electrostatic than Na. NaCl has 72%ionc character and CaF has 92% ionic charecter. No bond in chemistry is 100% ionic in nature.
Which rule states that the charge density of a cation is higher?
It can be explained by Fajan’s rule i.e for smaller charge,charge density on cation is higher; higher the size of cation & smaller size of the anion forms ionic compound and for higher charge , smaller the size of cation is more polarizable & distorted outermost electron cloud of higher size anion makes the bond covalent .
Which is more polar, Al3+ or Mg2+?from thestudentroom.co.uk
Al3+ is more polarising than Mg2+ and the Br- (higher charge + smaller ionic radius), the anion is large and polarisable.
Which direction does electronegativity increase?from thestudentroom.co.uk
You know that electronegativity increases from left to right and from down to up...