See more

How do you find the anomeric carbon?
In the cyclic form, the anomeric carbon can be found next to the oxygen atom in the pyranose or furanose ring, but on the opposite side from the carbon that carries the acyclic CH2O group (e.g., the CH2OH group in the example shown here).
Which carbon is an anomeric carbon?
In the cyclic form of sugar, the anomeric carbon is the carbon that was part of the carbonyl group in the straight-chain structure. When the chain converts to a ring, C−1 becomes a chiral center. C−1 is the anomeric carbon.
Which are the two anomeric forms of fructose?
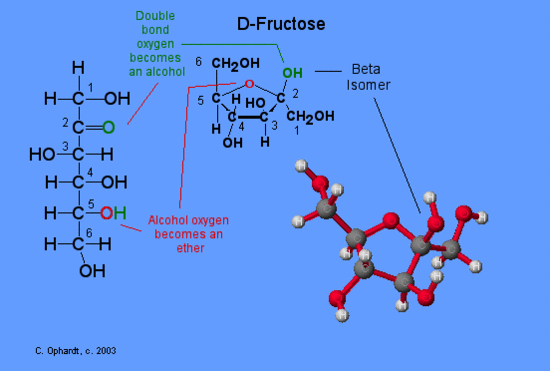
The carbon in position 1 is named anomeric and alpha and beta forms are said anomers. In the case of fructose the forms alpha and beta refers to the position of the hydroxyl group bound to anomeric carbon at position 2.
What is the anomeric carbon in sucrose?
Carbon # 1 (red on left) is called the anomeric carbon and is the center of an acetal functional group. A carbon that has two ether oxygens attached is an acetal. The Alpha position is defined as the ether oxygen being on the opposite side of the ring as the C # 6.
Is the anomeric carbon of fructose C2?
Solution : `C2` is the anomeric carbon in frustose. Since fructose contains a keto group , it forms an intramolecular hemiketal.
What is the anomeric carbon in glucose and fructose?
C−1 of glucose unit and C−2 of fructose unit are anomeric carbon atoms in the given disaccharide . the disaccharide is non-reducing sugar because −OH groups attached to anomeric carbon atoms are involved in the formation of glycosidic bond.
Is glucose and fructose anomers?
Glucose and fructose are not anomers.
Are alpha and beta fructose anomers?
The two anomers are described with the terms α (“alpha”) and β (“beta”), defined above. Anomeric carbon – the carbon of a cyclic sugar bearing a hemiacetal or acetal (hemiketal or ketal). This is C-1 in aldoses, and C-2 in the case of fructose.
What is anomeric carbon example?
The epimeric carbon in anomers are known as anomeric carbon or anomeric center. Example 1: α-D-Glucopyranose and β-D-glucopyranose are anomers. Example 2: α-D-Fructofuranose and β-D-fructofuranose are anomers. Example 3: Methyl α-D-glucopyranoside and methyl β-D-glucopyranoside are anomers.
What is the anomeric carbon in galactose?
Ring Structure for Galactose The anomeric carbon is the center of a hemiacetal functional group. A carbon that has both an ether oxygen and an alcohol group is a hemiacetal.
Which is the anomeric carbon in glucose?
C1 carbonIn carbohydrate chemistry, diastereomers differing only at the hemiacetal or acetal carbon are called anomers, and the hemiacetal or acetal carbon atom is called the anomeric carbon atom. 𝛼-form & 𝛽-form, are called anomers. Thus C1 carbon becomes the anomeric carbon of glucose in its pyranose form.
Where is the anomeric carbon in maltose?
The bonding between the glucopyranose rings in cellobiose and maltose is from the anomeric carbon in ring A to the C-4 hydroxyl group on ring B. This leaves the anomeric carbon in ring B free, so cellobiose and maltose both may assume alpha and beta anomers at that site (the beta form is shown in the diagram).
What is an anomeric carbon give an example?
An example of anomeric carbon is that carbon in a monosaccharide (like glucose) about which rotation occurs. The anomeric carbon can be determined by the carbon (C) attached to two oxygen (O) atoms joined by single bonds. This rotation brings about two distinct configurations, α and β -anomers.
What is an anomeric carbon quizlet?
The anomeric carbon is the carbon that has a carbonyl group in the open chain form.
What are examples of anomers?
Example 1: α-D-Glucopyranose and β-D-glucopyranose are anomers. Example 2: α-D-Fructofuranose and β-D-fructofuranose are anomers. Example 3: Methyl α-D-glucopyranoside and methyl β-D-glucopyranoside are anomers.
How do you identify anomers?
Anomers are special cases — they are epimers that differ in configuration only at the anomeric carbon. For example, α-D-glucose and β-D-glucose are anomers. The α form has the anomeric OH group at C-1 on the opposite side of the ring from the CH2OH group at C-5 .
What foods is fructose in?
Fructose is found in honey, sugar, fruit juices, candy and many more foods.
What does fructose do to your body?
Consumption of high quantities of fructose could lead to high blood sugar levels and heart disease.
What is the difference between glucose and fructose?
Glucose is a 6-membered ring whereas fructose is a 5-membered ring compound. Another important difference is glucose is aldohexose whereas fructose...
Is fructose a carbohydrate?
Yes, fructose is a naturally occurring carbohydrate sugar.
Is fructose a disaccharide or a monosaccharide?
Fructose is a monosaccharide carbohydrate sugar.
Anomers of Fructose Definition
An anomer is a kind of geometric variation; it creates in carbohydrate molecules of some atoms. Anomer is a Greek word, it means up, above. An epimer is a stereo isomer. It is different in the configuration at any stereogenic center. The epimeric carbon in the anomers is called anomeric carbon.
Overview of Anomers Of Fructose
Anomers are cyclic monosaccharides or glycosides. They are epimers. This epimers are different from each other, in the configuration of C − 1 {\rm {C - 1}} C −1 carbon, they are called aldoses or the configuration of C − 2 {\rm {C - 2}} C− 2 carbon, they are called ketoses. Anomerization is nothing but the conversion of one anomer to other anomer.
Alpha and beta anomers of Fructose
The anomeric hemiacetal carbon is involved in two carbon-carbon bonds to form a cyclic ring in fructose. In the open chain molecule, we open the molecule to form the anomeric carbon that converts into the keto carbonyl group. In fructose, they have two kinds of rings. It depends only on the point of hydroxyl group in fructose.
D and L configuration of Furanose ring
Cyclic hemiacetal of a ketohexose or an aldopentose are furanose ring. One oxygen atom and four carbon atoms through anomeric carbon in the direction of right side of the oxygen are present in furanose ring structure.
How many carbons are in fructose?
The fructose is identified as a five-membered ring having six-carbon, a hexose whereas glucose is a six-membered ring with –OH group on the carbon at 4 th position in a down projection.
What is the structure of fructose?
Structure of Fructose. Fructose has a cyclic or chair-like structure. The chair form of fructose is similar to that of glucose but in the structure of fructose, there are few exceptions. Fructose has a ketone functional group and the ring closure occurs from 2 nd carbon position. This result in the rise to 5-membered ring or there is a formation ...
What are the uses of fructose?
Some common uses of Fructose are: 1 It helps in enhancing the taste in food and beverages. 2 Crystalline fructose (natural sweetener) used in energy drinks, chocolate milk, cereals, and yogurts and many other beverages and low-calorie foods. 3 HFCS ( High Fructose Corn Syrup) products is a combination of glucose and fructose. 4 High fructose corn syrup is also used as a sweetener in many children and adult medicines such as cough suppressants and decongestant liquids.
What is HFCS in food?
HFCS ( High Fructose Corn Syrup) products is a combination of glucose and fructose.
What is high fructose corn syrup used for?
High fructose corn syrup is also used as a sweetener in many children and adult medicines such as cough suppressants and decongestant liquids.
Where is fructose found?
The fructose sugar is usually found naturally in fruits, flowers, trees, honey, berries and in rooted vegetables. As for the chemical properties, fructose is a monosaccharide and also a reducing sugar. The older name of fructose was levulose as it rotated the plane-polarized light in the left direction. This was the sole reason that it was ...
How many carbons are in a 5 member ring?
The five-membered ring has four carbons and one oxygen. There is basically a formation of chiral carbon and two arrangements of CH2OH and OH group. In essence, fructose displays stereoisomerism.
Anomeric center, The epimeric carbon
The anomeric center is also termed as the stereogenic center in stereoisomers. In carbohydrates, these stereoisomers are created by intermolecular cyclization through acetal or ketal groups. There exist two possibilities of the orientation of the hydroxyl group present on such carbon centers.
Anomeric carbon and Reducing sugars
The anomeric carbon, or to be very specific, the aldehydic carbon in aldoses and ketonic carbon in ketoses, has a hydroxyl group, which can be termed as anomeric hydroxyl. If that specific hydroxyl is not attached to any other structure, that sugar is a reducing sugar.
Anomerization
Anomerization is the process of conversion of one anomer to another. It is a spontaneous process that may sometimes involve acidic or basic catalysts. For reducing sugars, this anomerization is in fact mutarotation.
Anomeric effect
The anomeric effect or Edward Lemieux effect is the stereoelectronic effect describing the preference of axial orientation on the anomeric carbon, while the equatorial one is supposed to be the preferred one being less hindered. The magnitude of this anomeric effect is quantitated to be about 1.4 kcal/mol for OCO stabilization systems.
Examples of anomers (Anomeric carbon)
α-D glucose and β-D glucose, as pyranose forms i.e. [α-D glucopyranose and β-D glucopyranose]
Key Takeaway (s)
Anomers are special case epimers with differences in orientation of branched atoms at stereogenic centers.
Concepts Berg
The anomeric carbon can be identified as the carbon center which is an aldehydic or ketonic functional group carbon, in open-chain form.
