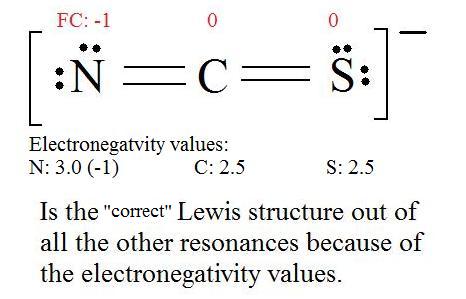What is the Lewis structure for OCN-?
For the Lewis structure for OCN- you should take formal charges into account to find the best Lewis structure for the molecule. Also note that you should put the OCN- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
How many lone pairs does OCN have?
OCN – (cyanate) has one oxygen atom, one carbon atom, and one nitrogen atom. In the lewis structure of OCN –, there is a single bond between carbon and oxygen atom, and a triple bond between carbon and nitrogen atom. The oxygen atom has three lone pairs, and the nitrogen atom has one lone pair.
What is the Lewis structure of CN in cyanide?
The nitrogen atom in cyanide ion, CN-, is surrounded by one triple bond and one lone pair of electrons. Formal charge is the difference between the number of valence electrons in a free atom and the number of electrons assigned to the atom in a Lewis structure. A Lewis structure of OCl- ion is drawn below.
What is the charge on the oxygen atom in OCN-Lewis structure?
Also, there is a negative (-1) charge on the oxygen atom. Here’s how you can draw the OCN – lewis structure step by step. Let’s break down each step in detail. In the periodic table, oxygen lies in group 16, carbon lies in group 14, and nitrogen lies in group 15.

What is the best Lewis structure for OCN?
1:122:06How to Draw the Lewis Structure for OCN- - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo this right here is the best lewis structure for ocn. Minus i'm dr b.MoreSo this right here is the best lewis structure for ocn. Minus i'm dr b.
How do you know which Lewis structure is more stable?
Typically, the structure with the most charges on the atoms closest to zero is the more stable Lewis structure.
Why is OCN stable?
OCN- lewis structure shape Also O and C have a single covalent bond and C and N atom has triple bond showing a stable form of structure. So, there are zero lone electron pairs on central carbon atom.
Is OCN stable?
The cyanate ion (OCN-) and the fulminate ion (CNO-) share the same three atoms but have vastly different properties. The cyanate ion is stable, while the fulminate ion is unstable and forms explosive compounds.
Which structure is most stable?
↑∴ octet complete.∴ most stable.
How do you determine if A molecule is stable or unstable?
Mechanical stability can be checked by computing the force constants (or the Hessian matrix of the energy second derivatives). If it is positive definite then your molecule is stable, meaning it will not deform as it is at its energy minimum. Chemical stability maybe checked by calculating the HOMO-LUMO gap.
Which is more stable CNO NCO?
1 Expert Answer. You're right that it has to do with formal charge. The less formal charges a molecule has, the more stable it is.
Is OCN linear or bent?
The total valence electron is 16 for drawing OCN– Lewis structure. In OCN– molecule, central carbon atom has zero lone pair and molecular geometry, as well as electron geometry, is linear. OCN– the molecule is polar in nature.
What is the molecular geometry of OCN?
This cyanate ion is stable. Its electron pair geometry is tetrahedral and the molecular geometry is trigonal pyramidal.
How many lone pairs are in the best Lewis structure of OCN -?
In the lewis structure of OCN–, there is a single bond between carbon and oxygen atom, and a triple bond between carbon and nitrogen atom. The oxygen atom has three lone pairs, and the nitrogen atom has one lone pair.
Which of the following resonance structures for OCN − will contribute most to the correct structure of OCN −?
Which of the following resonance structures for OCN- will contribute most to the correct structure of OCN-? e. They all contribute equally to the correct structure of OCN-.
What is the total number of valence electrons in the Lewis structure of OCN -?
For the OCN- Lewis structure there are a total of 16 valence electrons available.
Which type of calculation is best to find the most stable structure of a molecule?
Re: Most Stable Structure The best way to know when the Lewis structure is most stable is by calculating formal charge for each atom using FC= V-(S/2) where V are the valence electrons on that atom, and S is the number of shared electrons. The most stable lewis structure will have formal charges closest to 0.
How do you determine the polarity of a Lewis structure?
To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.
Which is stronger, ionic or covalent?
An ionic bond is much stronger than most covalent bonds.
Which type of bond has a lower potential energy than the two separate atoms?
A covalent bond has a lower potential energy than the two separate atoms.