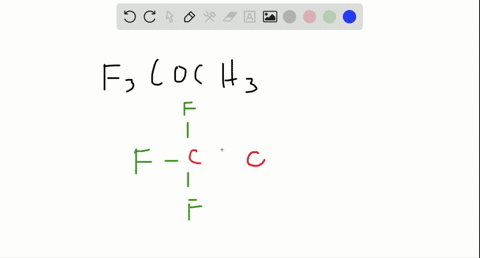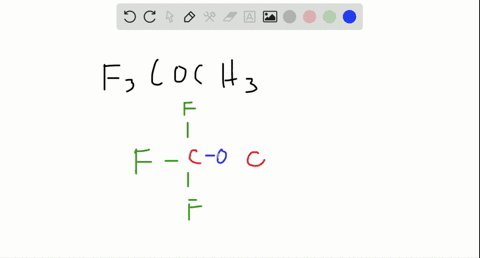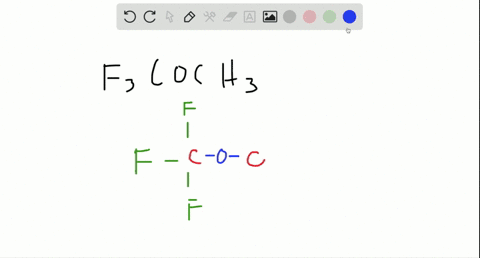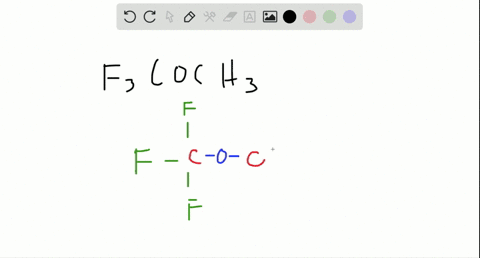
Are molecules only held together by covalent bonds?
Simple molecules contain only a few atoms held together by covalent bonds. An example is carbon dioxide (CO2), the molecules of which contain one atom of carbon bonded with two atoms of oxygen.
What molecule is formed by covalent bonding?
- Hydrogen (H 2) Both hydrogen atoms have only one electron, but by forming a single covalent bond, both can have a full outer shell.
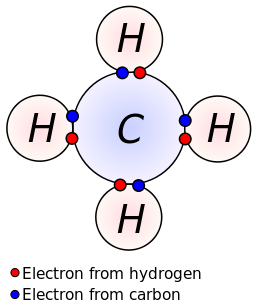
- Methane (CH 4)
- Ammonia (NH 3)
- Water (H 2 O)
What are 10 examples of covalent compounds?
What are 4 compounds from your everyday life that are covalent?
- Water.
- Sugar.
- Oxygen.
- Carbon Dioxide.
- LPG.
- Vinegar.
- Nail Polish Remover.
- Diamonds.
Are covalent and molecular bonds the same thing?
in covalent bonds electron sharing usually occurs so that atoms attain full outer energy levels. ... covalent and molecular. mean the same thing (nonmetals) so elements share. metals are. cations. nonmetals are. anions. covalent compounds names to formulas.

What molecules have single covalent bond?
Examples of molecules with single covalent bonds include hydrogen, chlorine, hydrogen chloride, methane, and all of the carbon-based biomolecules.
Is co2 a single covalent bond?
Carbon dioxide is made up of one carbon atom, two oxygen atoms. There are four covalent bonds in one molecule of carbon dioxide. Carbon and oxygen are non-metals, thus we know carbon dioxide is a covalent compound.
Is Cl2 a single covalent bond?
Yes, Cl2 is a single covalent bond. When two chlorine atoms share one electron, it is known as single covalent bond.
Which molecule has a single covalent bond quizlet?
Example: Hydrogen gas consists of diatomic molecules whose atoms share only one pair of electrons, forming a single covalent bond.
Is NH3 single bond?
A molecule of ammonia (NH3) has only single bonds.
Is H2O single or double bond?
i.e. H bonding with other molecules of water but there is no double bond in water molecule.
Is h2 a single covalent bond?
One carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons between itself and each hydrogen (H) atom....Properties of polar covalent bond:Number of electron pairs sharedType of covalent bond formed2Double3Triple1 more row
Does h2 have a single covalent bond?
Let's consider the covalent bond in the hydrogen molecule. A hydrogen molecule forms from two hydrogen atoms, each with one electron in a 1 s orbital. The two hydrogen atoms are attracted to the same pair of electrons in the covalent bond....Covalent Bonds.AtomValenceBromine1Chlorine1Iodine1Oxygen25 more rows
Is N2 a single covalent bond?
Nitrogen typically forms 3 covalent bonds, including in N2. This is because it has atomic number 7, so its electron configuration is 1s22s22p3, giving it 5 valence shell electrons.
What is a single covalent bond quizlet?
Single Covalent Bond. a bond formed when two atoms share a single pair of electrons.
What is a single bond made up of?
In chemistry, a single bond is a chemical bond between two atoms involving two valence electrons. That is, the atoms share one pair of electrons where the bond forms. Therefore, a single bond is a type of covalent bond.
How is a single covalent bond formed quizlet?
A bond formed when atoms share one or more pairs of electrons.
What type of covalent bond does CO2 have?
polar covalent bondThe type of bond between atoms in a molecule of CO2 is polar covalent bond. In carbon dioxide molecule, a carbon atom is joined by four covalent bonds to two oxygen atoms, which have two covalent bonds each.
Why is CO2 a double bond?
CO2 Double Bond Each O is surrounded by four dots and two sticks or lines, representing another 4 electrons in its double bond. So each O is surrounded by 8 total valence electrons, giving it an octet and making it stable. Carbon has four bonds, in this case present as two double bonds.
How many covalent bonds can CO2 form?
There are four covalent bonds in one molecule of carbon dioxide.
What is a single bond example?
Single bond symbol: A single line represents a bond between two atoms (i.e., involving one electron pair) Single bond example: Cl2, HCl, NH3 etc.
What are the characteristics of single covalent bonds?
One characteristic of single covalent bonds is a long bond length, the longest of any of the covalent bond types. Another characteristic is possibl...
What is an example of a single covalent bond?
An example of a single covalent bond is the bond between atoms in a water molecule. Oxygen shares one pair of electrons with two hydrogen atoms, fo...
What is a single covalent bond?
A single covalent bond is a type of chemical bond. In this bond type, two atoms share one pair of outer electrons to form an attraction.
What type of covalent bond is between two atoms?
Because they have the same electronegativity, they will share their valence electrons equally with each other. This type of a covalent bond where electrons are shared equally between two atoms is called a non-polar co valent bond.
Which element has only 2 valence electrons?
Exceptions to the octet rule include hydrogen (H) and helium (He) that follow the duet rule instead. They are the first two elements of the periodic table and have a single electron shell which accommodates only 2 electrons. Other exceptions include some group 3 elements like boron (B) that contain three valence electrons.
How many electrons does a nitrogen atom share with another nitrogen atom?
Diagram of single covalent bond being formed. Nitrogen atom can attain an octet configuration by sharing three electrons with another nitrogen atom, forming a triple bond (three pairs of electrons shared) Diagram of nitrogen bonding into octet configuration. Consider the molecule carbon dioxide (CO ).
Why are electrons not equally shared by the atoms?
This is due to the electronegativity difference between the two atoms. The more electronegative atom (Cl) has greater share of the electrons than the less electronegative atom (H).
What is the measure of how strongly an atom attracts electrons from another atom in a chemical bond?
Electronegativity is a measure of how strongly an atom attracts electrons from another atom in a chemical bond and this value is governed by where the particular atom is located in the periodic table (francium is the least electronegative element while fluorine is the most electronegative). Example of a covalent bond.
How many electrons are in an atom's outermost shell?
For any atom, stability is achieved by following the octet rule, which is to say all atoms (with a few exceptions) want 8 electrons in their outermost electron shell (just like noble gases). The electrons present in the outermost shell of an atom are called valence electrons.
What is the driving force for chemical bonding between atoms?
In general, achieving the octet configuration (i.e. 8 electrons in the outermost shell) is the driving force for chemical bonding between atoms. Take a look at the outer shell configuration (i.e. number of valence electrons) of three atoms – sodium (Na), chlorine (Cl) and neon (Ne):
Which structure only uses valence electrons in determining the bonding?
6. Lewis structures only use the valence electrons in determining the bonding.
What is the angle between the central carbon atom and the two oxygen atoms in CO2?
13. The VSEPR theory predicts that the angle between the central carbon atom and the two oxygen atoms in CO2 measures 180°.
Is NO a covalent compound?
c) NO is a pure covalent compound.