How can you determine if elements have the same chemical properties?
The easiest way to identify elements with similar chemical properties is to look at a periodic table. The periodic table begins by grouping chemicals into the following categories: metals, semimetals, and nonmetals. Most periodic tables will use the same colors to color code the elements into groups with the same chemical properties.
What determines the chemical behavior of elements?
What Determines the Chemical Behavior of an Atom?
- Atomic Structure. Atoms consist of three types of subatomic particle: protons, neutrons and electrons. ...
- Full Outer Energy Level. The number of electrons in an atom is determined by the number of protons. ...
- The Periodic Table. ...
- Ionisation Energy. ...
- Electron Affinity. ...
Do elements have different chemical characteristics?
Different elements have different melting and boiling points, and are in different states (liquid, solid, or gas) at room temperature. They also combine in different ways. Some form specific types of bonds, whereas others do not.
What determines the chemical reactivity or an element?
Reactivity is a measure of how easily an element will combine with other elements to form compounds. Some elements are unreactive and need energy putting in others will react spontaneously and easily. The size of the nucleus determines the chemical reactivity of the element due to its ability to hold onto electrons and attract electrons.
What are chemical properties?
How many isotopes does Europium have?
Is change in shape a physical property?
About this website

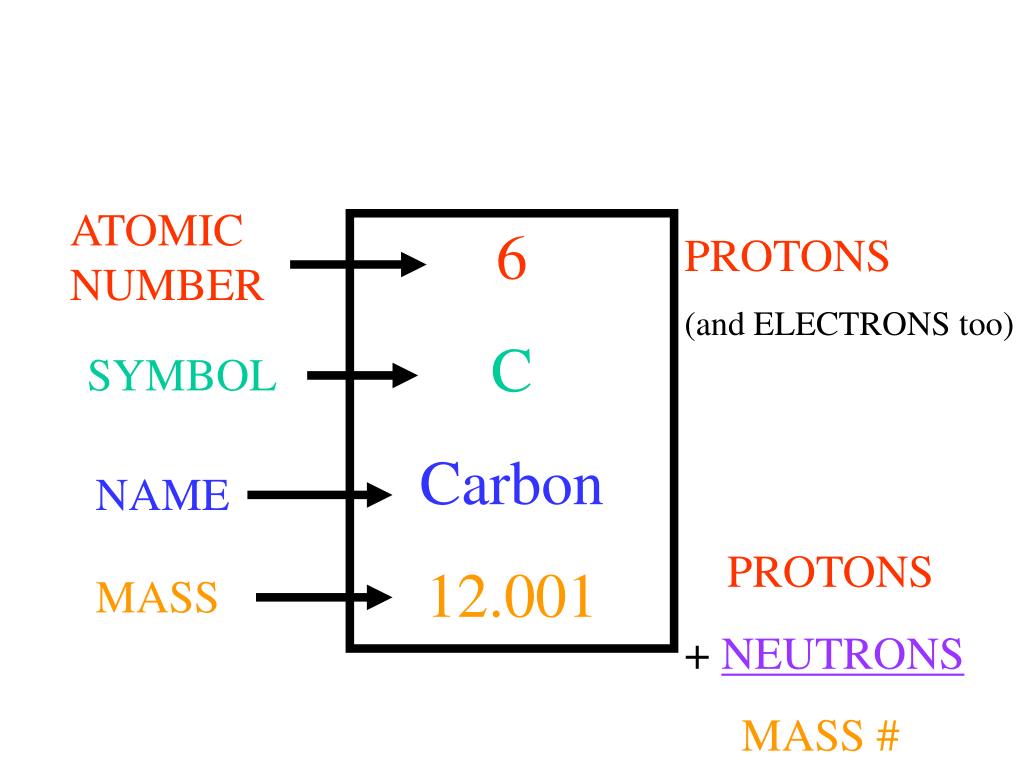
An element has the mass number 12 and atomic number 6. The number of ...
An element has the mass number 12 and atomic number 6. The number of neutron in it is? A)6 B)10 C)4 D)8 Get the answers you need, now!
9.65×10−12 - Brainly.com
What is 965,000,000,000,000 in scientific notation? - 5169901
Number of protons=Mass number=12 - Brainly
If an element M has mass number 24 and atomic number 12, how many neutrons does its atom contain and also find how many electrons and protons? - 43475251
Answer
What essentially determines the chemical properties of an element would be A. The number of and arrangement of the electrons in the atom.
Answer
The number and arrangement of the electrons in an atom is what determines the chemical properties of an atom. Chemical properties of an atom, element, or compound can be observed when a chemical change occurs, and the electrons surrounding an atom can help form chemical bonds with other atoms.
What are chemical properties?
Chemical properties are defined as the properties which tend to show change in chemical composition of a substance.
How many isotopes does Europium have?
URGENT!!! PLEASE HELP Europium has two naturally-occurring isotopes: europium-151 with an abundance of 47.82% and europium-153 with an abundance of 5 …
Is change in shape a physical property?
For example, change in shape, size, mass, volume etc are all physical properties.