What is post-translational modification in biology?
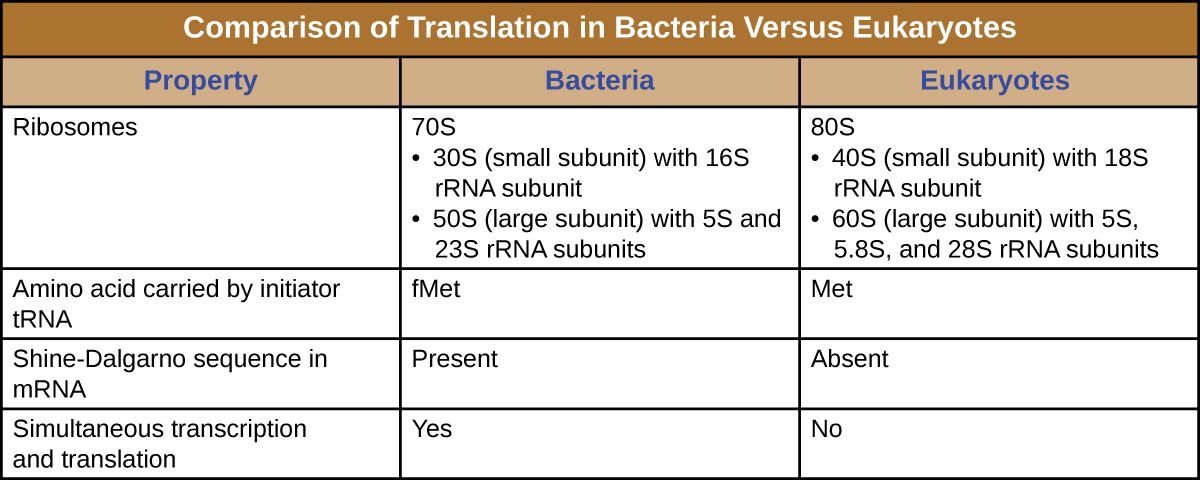
Post-translational modification. Post-translational modification ( PTM) refers to the covalent and generally enzymatic modification of proteins following protein biosynthesis. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product.
How do you modify a protein post translational?
Proteins are also covalently linked to tags that target a protein for degradation. They are modified through a combination of post-translational cleavage and the addition of functional groups through a step-wise mechanism of protein maturation or activation. Where does post translational modification occur?
Where do post-translational modifications (PTMs) occur?
PTMs occur at distinct amino acid side chains or peptide linkages, and they are most often mediated by enzymatic activity. Indeed, it is estimated that 5% of the proteome comprises enzymes that perform more than 200 types of post-translational modifications.
What are 3 types of post-translational modifications?
Types of post-translational modificationPhosphorylation.Acetylation.Hydroxylation.Methylation.
What occurs during post-translational modification quizlet?
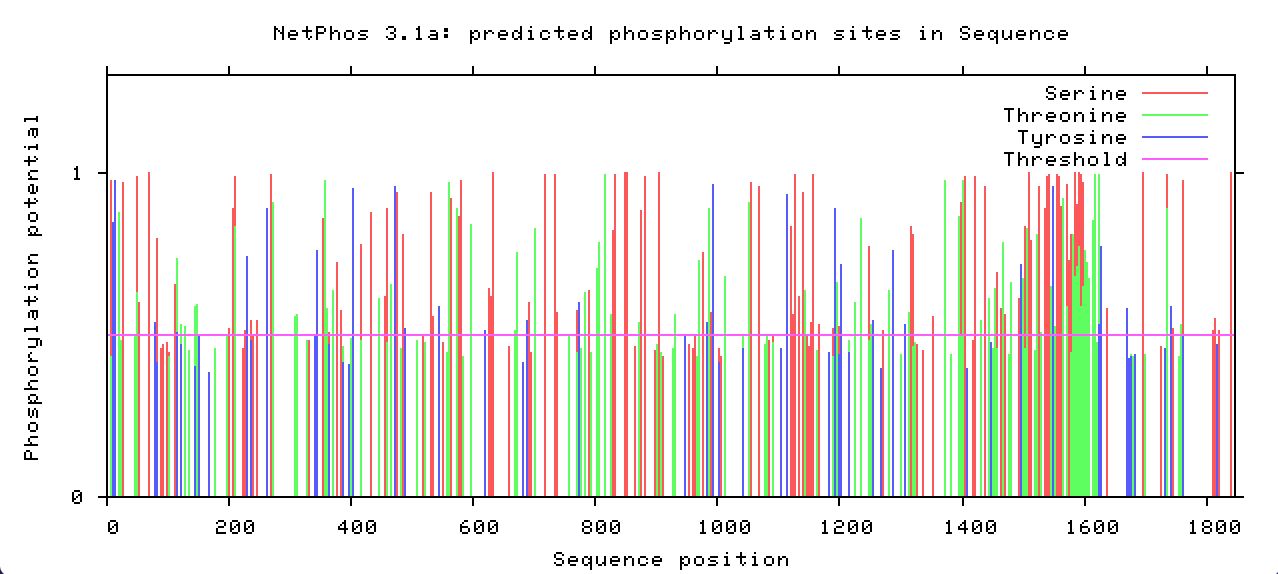
Post-translational modifications are changes to proteins that are made after translation has occurred. What are the amino acids that can be phosphorylated? Serine, tyrosine, and threonine. These are the only amino acids with a hydroxyl group.
Which of the following are post translational modifications of proteins?
These modifications include phosphorylation, glycosylation, ubiquitination, nitrosylation, methylation, acetylation, lipidation and proteolysis and influence almost all aspects of normal cell biology and pathogenesis.
Which of the following is not a type of post translational modification?
Which of the following is not a post-translational modification? Explanation: DNA methylation is not a post-translational modification. It is a biological process in which DNA molecules are methylated. Lipidation, protein phosphorylation, and proteolytic processing are proteolytic processing.
Which sites undergo post-translational modification?
Sites that often undergo post-translational modification are those that have a functional group that can serve as a nucleophile in the reaction: the hydroxyl groups of serine, threonine, and tyrosine; the amine forms of lysine, arginine, and histidine; the thiolate anion of cysteine; the carboxylates of aspartate and glutamate; and the N- and C-termini. In addition, although the amide of asparagine is a weak nucleophile, it can serve as an attachment point for glycans. Rarer modifications can occur at oxidized methionines and at some methylenes in side chains.
What is the post-translational modification of insulin?
At the top, the ribosome translates a mRNA sequence into a protein, insulin, and passes the protein through the endoplasmic reticulum, where it is cut, folded and held in shape by disulfide (-S-S-) bonds. Then the protein passes through the golgi apparatus, where it is packaged into a vesicle. In the vesicle, more parts are cut off, and it turns into mature insulin.
What is diphthamide formation?
diphthamide formation (on a histidine found in eEF2) ethanolamine phosphoglycerol attachment (on glutamate found in eEF1α) hypusine formation (on conserved lysine of eIF5A (eukaryotic) and aIF5A (archaeal)) beta-Lysine addition on a conserved lysine of the elongation factor P (EFP) in most bacteria.
What happens to a protein after disulfide bonds are formed?
For instance, the peptide hormone insulin is cut twice after disulfide bonds are formed, and a propeptide is removed from the middle of the chain; the resulting protein consists of two polypeptide chains connected by disulfide bonds. Some types of post-translational modification are consequences of oxidative stress.
What is the term for attaching a lipid to a protein?
Attachment of lipid molecules, known as lipidation , often targets a protein or part of a protein attached to the cell membrane . Other forms of post-translational modification consist of cleaving peptide bonds, as in processing a propeptide to a mature form or removing the initiator methionine residue. The formation of disulfide bonds ...
What is PTM in vesicle?
In the vesicle, more parts are cut off, and it turns into mature insulin. Post-translational modification ( PTM) refers to the covalent and generally enzymatic modification of proteins following protein biosynthesis. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form ...
Where do PTMs occur?
Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini.
Where does post translational modification occur?
Where does post translational modification occur? PTMs occur at distinct amino acid side chains or peptide linkages and are most often mediated by enzymatic activity. Indeed, 5% of the proteome comprises enzymes that perform more than 200 types of PTMs (4). These enzymes include kinases, phosphatases, transferases, and ligases, which add or remove functional groups, proteins, lipids, or sugars to or from amino acid side chains, and proteases, which cleave peptide bonds to remove specific sequences or regulatory subunits. Many proteins can also modify themselves using autocatalytic domains, such as autokinase and autoprotolytic domains. PTMs can also be reversible based on the nature of the modification. As an example, phosphatases hydrolyze the phosphate group to remove it from the protein and reverse its biological activity (Figure 1).
What is the most commonly studied post-translational modification?
Protein phosphorylation (Figure 2) is the most commonly studied post-translational modification. It has been estimated that one-third of mammalian proteins may be phosphorylated, and this modification often plays a key role in modulating protein function.
What is ubiquitination in biology?
Ubiquitin is a small (+/-8.6 kDa) protein expressed across almost all tissue types (Figure 3). Ubiquitination is an enzymatic reaction catalyzed by a three-enzyme cascade (E1, E2, and E3). That provides substrate specificity and activation, conjugation, and ligation steps. Proteins can be monoubiquitinated (with one ubiquitin molecule) or polyubiquitinated. Polyubiquitination takes place when additional ubiquitin molecules are added to the initial ubiquitin molecule. Ubiquitination via the proteome can mark proteins for degradation. It is also important for cellular signaling, the internalization of membrane proteins , and the development and regulation of transcription.
How many types of PTMs are there?
Consequently, novel enrichment strategies have uncovered the global cellular importance of several types of modifications (e.g., acetylation, ubiquitylation, O-GlNac, N-linked glycosylation). More than 200 diverse types of PTMs are currently known (5,6), ranging from small chemical modifications (e.g., phosphorylation and acetylation) to the addition of complete proteins (e.g., ubiquitylation, Figure 3).
What is the role of PTMs in cellular processes?
PTMs play an important part in modifying the end product of expression , contribute to biological processes and diseased conditions, playing a key role in many cellular processes such as cellular differentiation (1), protein degradation, signaling and regulatory processes, regulation of gene expression, and protein-protein interactions (2,3).
Why is the analysis of PTMs important?
The analysis of proteins and their PTMs is particularly important for the study of heart disease, cancer, neurodegenerative diseases, and diabetes (7) . The main challenges in studying post-translationally modified proteins are the development of specific detection and purification methods.
What does PTM mean in biology?
Conditional chemical changes are relayed from sensors to effectors via reversible post-translational modifications (PTMs) of proteins.
When does PTM occur?
PTM can occur at any stage of the protein life. Some proteins are modified shortly after their translation is completed and prior to the final steps of their folding.
What is the role of PTMs in the cell?
Posttranslational modifications (PTMs) of proteins perform crucial roles in regulating the biology of the cell. PTMs are enzymatic, covalent chemical modifications of proteins that typically occur after the translation of mRNAs.
What are PTMs in biology?
Posttranslational modifications (PTMs) of proteins such as phosphorylation and ubiquitination are crucial for controlling protein stability, localization, and conformation. Genetic information encoded in DNA is transcribed, translated, and increases its complexity by multiple PTMs. Conformational change introduced by PTMs affects interacting partners of each proteins and their downstream signaling; therefore, PTMs are the major level of modulations of total outcome of living cells. Plants are living in harsh environment that requires unremitting physiological modulation to survive, and the plant response to various environment stresses is regulated by PTMs of proteins. This review deals with the novel knowledge of PTM-focused proteomic studies on various life conditions. PTMs are focused that mediate plant–environment interaction such as stress perception, protein homeostasis, control of energy shift, and defense by immune system. Integration of diverse signals on a protein via multiple PTMs is discussed as well, considering current situation where signal integration became an emerging area approached by systems biology into account.
What is the role of O-linked N-acetylglucosaminylation (O-?
In particular, O-linked N-acetylglucosaminylation (O-GlcNAc) has emerged as a critical dynamic PTM of a wide range of cytosolic, nuclear, and mitochondrial proteins. 207–209 Importantly, O-GlcNAc of various proteins plays an essential role in cardiovascular physiology and pathophysiology and heart aging. 210–213 Despite some difficulties in quantitatively detecting this PTM, modern advanced MS technologies allow for simultaneously identifying and localizing both O-GlcNAc and phosphorylation sites in hundreds of proteins. 214,215 Functional interplay between different PTMs is emerging as an important topic in proteomics, which further stimulates refinement of MS-based proteomic technologies.
What is a PTM in protein?
Post-translational modifications (PTMs) are widespread and have important roles in the regulation of many protein functions. This article highlights factors to consider when developing, evaluating or using tools for PTM prediction. These include curation of modification datasets, to help manage false positives and negatives, and the use of enzyme-specific data whenever possible. This article also explores the utility of predicted PTMs and the challenges associated with predicting the function of modification sites. We note that many PTMs are increasingly associated with protein-protein interaction interfaces, raising the prospect that PTMs may be involved in complicated combinations that constitute a protein ‘interaction code’.
How many PTMs are there?
More than 40 PTMs have been identified and related to diseases such as cancer and neurological disorders. These PTMs play a major role in protein folding, stability, conformation, etc. Of all the post-translational modifications, phosphorylation and glycosylation are the major players in many of the protein functions.
Why are PTMs important?
These modifications are relevant because they can potentially change a protein's physical or chemical properties, activity, localization, or stability. Some PTMs can be added and removed dynamically as a mechanism for reversibly controlling protein function and cell signaling.
What is post translational modification?
Post translational modifications refer to any alteration in the amino acid sequence of the protein after its synthesis. It may involve the modification of the amino acid side chain, terminal amino or carboxyl group by means of covalent or enzymatic means following protein biosynthesis. Generally, these modifications influence the structure, ...
Where does sulfate modification occur?
Sulfate modidication takes place by the addition of sulphate molecules and these modifications of proteins occurs at tyrosine residues. Tyros ine sulfation accomplished via the activity of tyrosylproteinsulfotransferases (TPST) which are membrane associated enzymes of trans-Golgi network. There are two known TPSTs. TPST-1 TPST-2 The universal phosphate donor is 3’-phosphoadenosyl- 5’-phosphosulphate (PSPA).
How does glycosylation affect stability?
Confers stability to the protein- glycosylation can modify the stability of the protein by increasing protein half life.
What is the process of adding hydroxyl groups to amino acids?
Hydroxylation. The biological process of addition of a hydroxy group to a protein amino acid is called Hydroxylation. Protein hydroxylation is one type of PTM that involves the conversion of –CH group into –COH group and these hydroxylated amino acids are involved in the regulation of some important factors called transcription factors.
What is the transfer of one carbon methyl groups to nitrogen or oxygen to amino acid side chains?
The transfer of one-carbon methyl groups to nitrogen or oxygen to amino acid side chains increases the hydrophobicity of the protein and can neutralize a negative amino acid charge when bound to carboxylic acids. Methylation is mediated by methyltransferases and S-adenosyl methionine (SAM) is the primary methyl group donor.
What is a SUMOylation protein?
a) SUMOylation. SUMO (small ubiquitin related modifier) proteins are 100 amino acid residue proteins which bind to the target protein in the same way as ubiquitin. They also confer the transcription regulatory activity of the protein and help in the transport of the target protein from cytosol to the nucleus.
Why are PTMs important?
PTMs are important components in cell signaling, as for example when prohormones are converted to hormones.
What is post translational modification?
Post-translational modifications are the chemical modifications a polypeptide chain receives after it is translated that convert it to the mature protein. A protein is made up of a chain of amino acids, also known as a polypeptide. During translation, 20 different amino acids can be incorporated to make up a polypeptide chain.
What are the post-translational modifications that involve the addition of a functional group to a protein?
Some very common post-translational modifications that involve the addition of a functional group to a protein include glycosylation, lipidation, ubiquitination, and phosphorylation. Proteins that are destined to be embedded in the plasma membrane often undergo glycoslylation or lipidation.
Why do enzymes cut polypeptide chains?
It may be necessary for enzymes to cut the polypeptide chain to remove single amino acids or even entire regions of the polypeptide before it can become a functional protein. For example, the first amino acid in a polypeptide (methionine) is often removed, as this usually corresponds to the start codon that initiated translation. ...
What is the difference between lipidation and glycosylation?
Glycosylation describes the addition of a carbohydrate group to a protein, which can act as a cell surface marker, while lipidation describes the addition of a lipid to a protein, which can help anchor that protein to the cell membrane.
What is the term for the breakdown of a protein into polypeptides?
Disulfide bonds: A covalent bond formed between the thiol groups in two cysteine amino acids. Proteolysis: The breakdown of a protein into polypeptides, usually carried out by an enzyme.
What is the name of the process of adding a carbohydrate to a protein?
Glycosy lation: The addition of a carbohydrate group to a protein. Lipidation: The addition of a lipid to a protein. Ubiquitination: The addition of a ubiquitin protein to another protein. Phosphorylation: The addition of a phosphoryl group to a protein. Proteasome: Protein complex in the cell that degrades proteins.
What is the function of ubiquitination?
In ubiquitination, the protein ubiquitin is appended to another protein of interest, marking it for degradation by the proteasome. Protein degradation occurs when there are damaged proteins, or as a normal part of regulating protein activity in the cell. Phosphorylation and dephosphorylation are common modifications that are used in biochemical ...

Overview
Post-translational modification (PTM) refers to the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important co…
PTMs involving addition of functional groups
• myristoylation (a type of acylation), attachment of myristate, a C14 saturated acid
• palmitoylation (a type of acylation), attachment of palmitate, a C16 saturated acid
• isoprenylation or prenylation, the addition of an isoprenoid group (e.g. farnesol and geranylgeraniol)
Conjugation with other proteins or peptides
• ubiquitination, the covalent linkage to the protein ubiquitin.
• SUMOylation, the covalent linkage to the SUMO protein (Small Ubiquitin-related MOdifier)
• neddylation, the covalent linkage to the Nedd protein
Chemical modification of amino acids
• citrullination, or deimination, the conversion of arginine to citrulline
• deamidation, the conversion of glutamine to glutamic acid or asparagine to aspartic acid
• eliminylation, the conversion to an alkene by beta-elimination of phosphothreonine and phosphoserine, or dehydration of threonine and serine
Structural changes
• disulfide bridges, the covalent linkage of two cysteine amino acids
• proteolytic cleavage, cleavage of a protein at a peptide bond
• isoaspartate formation, via the cyclisation of asparagine or aspartic acid amino-acid residues
Databases and tools
Protein sequences contain sequence motifs that are recognized by modifying enzymes, and which can be documented or predicted in PTM databases. With the large number of different modifications being discovered, there is a need to document this sort of information in databases. PTM information can be collected through experimental means or predicted from high-quality, manuall…
See also
• Protein targeting
• Post-translational regulation
External links
• dbPTM - database of protein post-translational modifications
(Wayback Machine copy)
• List of posttranslational modifications in ExPASy
• Browse SCOP domains by PTM — from the dcGO database
A Brief Overview
How Does Post Translational Modification Work?
- PTMs can happen at any step of the protein lifespan. Many proteins are modified shortly after translation is completed to mediate proper folding or to direct the nascent protein to distinct cellular locations (such as the nucleus or membrane). Other modifications occur after folding and localization are completed to activate or inactivate catalytic...
Most Common Post-Translational Modifications
- Recent developments in mass-spectrometry (MS) methods have enabled the identification of thousands of PTM sites. Consequently, novel enrichment strategies have uncovered the global cellular importance of several types of modifications (e.g., acetylation, ubiquitylation, O-GlNac, N-linked glycosylation). More than 200 diverse types of PTMs are currently known (5,6), ranging fr…
Ptms Impact on Health and Disease
- The analysis of proteins and their PTMs is particularly important for the study of heart disease, cancer, neurodegenerative diseases, and diabetes (7). The main challenges in studying post-translationally modified proteins are the development of specific detection and purification methods. Fortunately, these technical obstacles are being overcome with a variety of new and re…