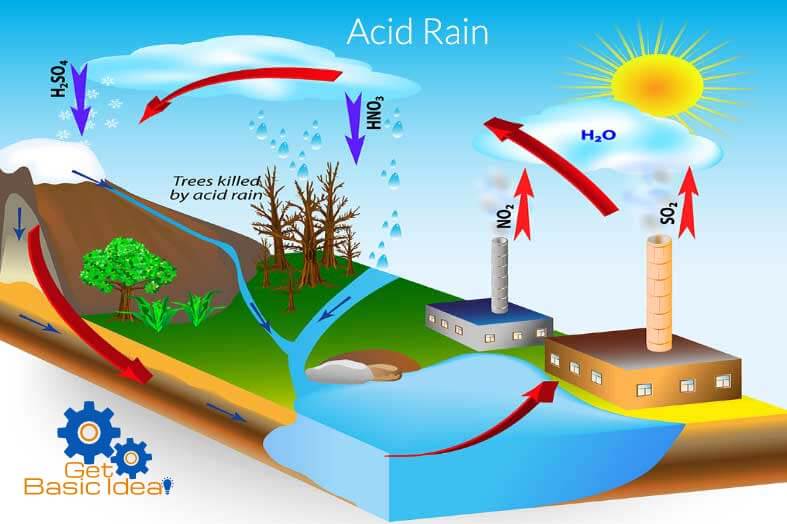
Is acid rain a real thing?
Originally Answered: Is acid rain is real? Yes, it is. The term 'acid rain' was first used by Robert Angus in 1872. Literally it means the presence of excessive acids in rain water. It is in fact cocktail of mainly H2SO4 and HNO3, where the ratio of these two may vary depending upon the relative quantities of oxides of sulphur and nitrogen emitted.
What are facts about acid rain?
Prevention of Acid Rain
- The only precaution that we can take against acid rain is having a check at the emission of oxides of nitrogen and sulphur.
- Acid rain is harmful to animals, plants and the monuments.
- Being responsible citizens, one should be aware of the harmful effects they cause and of the industries which give out nitrogen and sulphur compound wastes unethically.
What causes smog and acid rain?
What are the two main causes of air pollution?
- The Burning of Fossil Fuels. …
- Agricultural Activities. …
- Waste in Landfills. …
- Exhaust From Factories and Industries. …
- Mining Operations. …
- Indoor Air Pollution. …
- Natural Events.
How does acid rain occur naturally?
- Causes of acid rain. Sources/Usage: Public Domain. ...
- Effects of acid rain. The environment can generally adapt to a certain amount of acid rain. ...
- Geographic distribution of acid rain. Acidity in rain is measured by collecting samples of rain and measuring its pH. ...
- Acid rain and stone. ...

Does pollution cause acid rain?
Acid rain is one of the consequences of air pollution. Gases produced from the burning of fuels react with the oxygen in the air and water vapour, transforming into acids that fall onto the earth's surface as rain.
Which type of pollution can cause acid rain in India?
The burning of fossil fuels is mainly responsible for creation of sulphur dioxide ( so 2 ) and oxides of nitrogen ( no x ) which lead to the formation of acid rain. Automobile exhaust fumes are partly to blame, but the worst culprits are coal-burning thermal power plants and the steel industry.
What is the main cause of acid rain?
Causes of acid rain The biggest sources are coal-burning power plants, factories, and automobiles. When humans burn fossil fuels, sulfur dioxide (SO2) and nitrogen oxides (NOx) are released into the atmosphere.
Is acid rain caused by carbon dioxide?
Because carbonic acid is a relatively weak acid, the ability of carbon dioxide alone to generate true “acid rain” is very limited. Acid rain is caused by industrial emissions of sulfur dioxide and nitrogen oxides (which form much stronger acids when equilibrated in rainwater).
How acid rain occur in India?
The scientists cite the example of northeast India where oil refineries, fertiliser factories, thermal power plant and oil and gas installations release sulphate and nitrate compounds into the atmosphere, raising acid content in the rain. There are other reasons too.
Where does acid rain occur in India?
Analysis of rainwater samples from Nagpur, Mohanbari (in Assam), Allahabad, Visakhapatnam and Kodaikanal in the decade 2001-2012 showed a pH level varying from 4.77 to 5.32, indicating that these places have actually been receiving 'acid rain'. Rainwater with pH below 5.65 is considered acidic.
When was the first acid rain in India?
In 1974 study was conducted at Trombay in Mumbai which resulted in the release of the first report on acid rain in India. Scientists observed the high incidence of acidic and sulphate compounds in the air.
Which country has the most acid rain?
Acid rain erodes most building materials as well as crops, leading eventually to human consumption. Rain traditionally slacks off from September on. China is the world's biggest sulfur dioxide polluter, with 25.49 million tons discharged in 2005, up 27 percent from 2000.
What causes acid rain?
The main sources of pollutants that trigger acid rain are vehicles and industrial and power-generating plants. The areas of greatest acidity are in the northeastern United States.
How does acid rain affect the environment?
IMPACT OF ACID RAIN ON FORESTS. Acid rain can dissolve certain more soluble elements from the soil, like aluminum. The dissolved aluminum begins to accumulate and can reach toxic levels as it enters local streams and wetlands. Acid rain also removes important nutrients from the soil, such as calcium, potassium, and magnesium.
What is the National Acid Precipitation Assessment Program?
The National Acid Precipitation Assessment Program (NAPAP), a Federal program involving representatives from more than a dozen Federal agencies, has sponsored studies on how acid rain forms and how it affects lakes, crops, forests, and materials.
What is acid rain?
Acid rain is the term commonly used by scientists to describe rain that is abnormally acidic. What does that mean? Well, plain distilled water, like that used in laboratories, is neutral (not acidic or basic). Since rain naturally has things dissolved in it, it will always be slightly acidic.
What are the hazards of volcanic air pollution in Hawaii?
Volcanic air pollution hazards in Hawaii. Noxious sulfur dioxide gas and other air pollutants emitted from Kīlauea Volcano on the Island of Hawai‘i react with oxygen, atmospheric moisture, and sunlight to produce volcanic smog (vog) and acid rain.
Why are historic buildings affected by acid rain?
Because buildings and monuments cannot adapt to changes in the environment, as plants and animals can, historic structures may be particularly affected by acid precipitation. Scientists are studying effective control technologies to limit the emissions from power plants and automobiles that cause acid rain.
Why are forests more susceptible to pests, disease, and injury from freezing and drought?
Lastly, the combination of reduced calcium and excessive aluminum can make forests more susceptible to pests, disease, and injury from freezing and drought, as a proper balance of these nutrients is vital to forest health. A forest of dead trees damaged by acid rain (Credit: Pixabay).
What is the result of acid rain?
Acid rain results when sulfur dioxide (SO 2) and nitrogen oxides (NO X) are emitted into the atmosphere and transported by wind and air currents. The SO 2 and NO X react with water, oxygen and other chemicals to form sulfuric and nitric acids. These then mix with water and other materials before falling to the ground.
What is acid rain?
Acid rain, or acid deposition, is a broad term that includes any form of precipitation with acidic components, such as sulfuric or nitric acid that fall to the ground from the atmosphere in wet or dry forms. This can include rain, snow, fog, hail or even dust that is acidic.
How does acidic water affect the environment?
When the accumulated acids are washed off a surface by the next rain, this acidic water flows over and through the ground, and can harm plants and wildlife, such as insects and fish.
Where does acid rain come from?
While a small portion of the SO 2 and NO X that cause acid rain is from natural sources such as volcanoes, most of it comes from the burning of fossil fuels. The major sources of SO 2 and NO X in the atmosphere are: Burning of fossil fuels to generate electricity.
Is rain acidic or alkaline?
The lower a substance's pH (less than 7), the more acidic it is; the higher a substance's pH (greater than 7), the more alkaline it is. Normal rain has a pH of about 5.6; it is slightly acidic because carbon dioxide (CO 2) dissolves into it forming weak carbonic acid. Acid rain usually has a pH between 4.2 and 4.4.
What is the cause of acid rain?
Acid rain is caused by a chemical reaction that begins when compounds like sulfur dioxide and nitrogen oxides are released into the air. These substances can rise very high into the atmosphere, where they mix and react with water, oxygen, and other chemicals to form more acidic pollutants, known as acid rain.
What happens if precipitation becomes too acidic?
However, if precipitation becomes too acidic, these materials may not be able to neutralize all of the acids. Over time, these neutralizing materials can be washed away by acid rain. Damage to crops, trees, lakes, rivers, and animals can result.
Is rain acidic or non acidic?
Nature depends on balance, and although some rain is naturally acidic, with a pH level of around 5.0, human activities have made it worse. Normal precipitation —such as rain, sleet, or snow—reacts with alkaline chemicals, or non-acidic materials, that can be found in air, soils, bedrock, lakes, and streams.
How does acid rain affect aquatic life?
Acid rain has many ecological effects, especially on lakes, streams, wetlands, and other aquatic environments. Acid rain makes such waters more acidic, which results in more aluminum absorption from soil, which is carried into lakes and streams. That combination makes waters toxic to crayfish, clams, fish, and other aquatic animals.
How does acid rain affect trees?
The pollutants may also inhibit trees' ability to reproduce. Some soils are better able to neutralize acids than others.
What is the pH of acid rain?
Acid rain describes any form of precipitation that contains high levels of nitric and sulfuric acids. It can also occur in the form of snow, fog, and tiny bits of dry material that settle to Earth. Normal rain is slightly acidic, with a pH of 5.6, while acid rain generally has a pH between 4.2 and 4.4.
What happens when you burn fossil fuels?
When humans burn fossil fuels, sulfur dioxide ( SO 2) and nitrogen oxides (NO x) are released into the atmosphere. Those air pollutants react with water, oxygen, and other substances to form airborne sulfuric and nitric acid. Winds may spread these acidic compounds through the atmosphere and over hundreds of miles.
What is the Clean Air Act?
This means burning fewer fossil fuels and setting air-quality standards. In the U.S., the Clean Air Act of 1990 targeted acid rain, putting in place pollution limits that helped cut sulfur dioxide emissions 88 percent between 1990 and 2017. Air-quality standards have also driven U.S. emissions of nitrogen dioxide down 50 percent in ...
Why are nitrogen oxides harmful?
But nitrogen oxides contribute to the formation of ground-level ozone, a major pollutant that can be harmful to people. Both gases cause environmental and health concerns because they can spread easily via air pollution and acid rain.
Why are trees hard to take up water?
The acid deposits rob the soil of essential nutrients such as calcium and cause aluminum to be released in the soil, which makes it hard for trees to take up water. Trees' leaves and needles are also harmed by acids. The effects of acid rain, combined with other environmental stressors, leave trees and plants less healthy, ...
How does acid rain affect ecosystems?
The Effects of Acid Rain on Ecosystems. This figure illustrates the pH level at which key organisms may be lost as their environment becomes more acidic. Not all fish, shellfish, or the insects that they eat can tolerate the same amount of acid. An ecosystem is a community of plants, animals and other organisms along with their environment ...
What happens to trees when they get acid rain?
Acid rain also removes minerals and nutrients from the soil that trees need to grow. At high elevations, acidic fog and clouds might strip nutrients from trees’ foliage, leaving them with brown or dead leaves and needles.
What is episodic acidification?
Episodic Acidification. Melting snow and heavy rain downpours can result in what is known as episodic acidification. Lakes that do not normally have a high level of acidity may temporarily experience effects of acid rain when the melting snow or downpour brings greater amounts of acidic deposition and the soil can’t buffer it.
What gases can be transformed into sulfate and nitrate particles?
In the atmosphere, SO 2 and NO X gases can be transformed into sulfate and nitrate particles, while some NO X can also react with other pollutants to form ozone. These particles and ozone make the air hazy and difficult to see through.
What happens when acidic particles corrode metal?
The acidic particles corrode metal and cause paint and stone to deteriorate more quickly. They also dirty the surfaces of buildings and other structures such as monuments. The consequences of this damage can be costly: loss of detail on stone and metal statues, monuments and tombstones.
What are the effects of sulfur dioxide on the heart?
Many scientific studies have shown a relationship between these particles and effects on heart function, such as heart attacks resulting in death for people with increased heart disease risk, and effects on lung function, such as breathing difficulties for people with asthma. Learn more about: Sulfur Dioxide. Nitrogen Oxides.
Does acid rain cause pollution?
Nitrogen Pollution. It’s not just the acidity of acid rain that can cause problems. Acid rain also contains nitrogen, and this can have an impact on some ecosystems. For example, nitrogen pollution in our coastal waters is partially responsible for declining fish and shellfish populations in some areas.
What happens when acid rains?
Acid rain also causes the corrosion of water pipes. Which further results in leaching of heavy metals such as iron, lead and copper into drinking water. It damages the buildings and monuments made up of stones and metals.
How does acid rain affect agriculture?
Acid rain affects agriculture by the way how it alters the composition of the soil. It causes respiratory issues in animals and humans. When acid rain falls down and flows into the rivers and ponds it affects the aquatic ecosystem. As it alters the chemical composition of the water, to a form which is actually harmful to ...
What is the impact of acid rain on Taj Mahal?
The city of Agra has many industries which emit the oxides of sulphur and nitrogen in the atmosphere. People continue to use low-quality coal and firewood as a domestic fuel, adding to this problem. Acid rain has the following reaction with the marble ( calcium carbonate ):
What is acid rain made of?
Acid rain is made up of highly acidic water droplets due to air emissions, most specifically the disproportionate levels of sulphur and nitrogen emitted by vehicles and manufacturing processes. Often called acid rain as this concept contains many types of acidic precipitation. The acidic deposition takes place in two ways: wet, and dry.
How can we prevent acid rain?
Prevention of Acid Rain 1 The only precaution that we can take against acid rain is having a check at the emission of oxides of nitrogen and sulphur. 2 We have so far seen the details of acid rain and its harmful effect on animals, plants and the monuments. 3 Being responsible citizens, one should be aware of the harmful effects they cause and of the industries which give out nitrogen and sulphur compound wastes unethically.
How are sulphur and nitrogen mixed?
Sulphur and Nitrogen particles which get mixed with water are found in two ways either man-made i.e as the emissions are given out from industries or by natural causes like how a lightning strike in the atmosphere releases nitrogen ions and sulphur is released from volcanic eruptions.
What are some examples of oxidation reactions?
Example – the burning of fossil fuels, unethical waste emission disposal techniques . Sulphur dioxide and nitrogen dioxide undergo oxidation, and then they react with water resulting in the formation of sulphuric acid and nitric acid respectively. The following reaction will clarify the acid formation reaction:
Why is acid rain acidic?
our editorial process. Amanda Briney. Updated January 23, 2020. Acid rain is made up of water droplets that are unusually acidic because of atmospheric pollution, most notably the excessive amounts of sulfur and nitrogen released by cars and industrial processes.
Why is acid falling on trees harmful?
Acid falling on a forest’s soil is also harmful because it disrupts soil nutrients, kills microorganisms in the soil, and can sometimes cause a calcium deficiency.
How does acid deposition affect architecture?
Finally, acid deposition also has an effect on architecture and art because of its ability to corrode certain materials. As acid lands on buildings (especially those constructed with limestone), it reacts with minerals in the stones, sometimes causing them to disintegrate and wash away.
Why do acids disperse over large areas?
These acids then disperse over large areas because of wind patterns and fall back to the ground as acid rain or other forms of precipitation. The gases most responsible for acid deposition are a byproduct of electric power generation and the burning of coal.
What is the most affected by acid deposition?
Aquatic settings are the most clearly affected by acid deposition, however, because acidic precipitation falls directly into them. Both dry and wet deposition also runs off from forests, fields, and roads and flows into lakes, rivers, and streams. As this acidic liquid flows into larger bodies of water, it is diluted.
How many lakes have a pH below normal?
It is estimated that around 50,000 lakes in the United States and Canada have a pH below normal (about 5.3 for water). Several hundred of these have a pH too low to support any aquatic life. Aside from aquatic bodies, acid deposition can significantly affect forests.
What is the pH of rainwater?
Normal rainwater is slightly acidic, with a pH range of 5.3-6.0. Acid deposition is anything below that range. It is also important to note that the pH scale is logarithmic, and each whole number on the scale represents ...
What are the causes of acid rain?
Forms of Acid Rain. Wet Deposition: This is the most common form of acid rain. The sulphuric acid and nitric acid are the main causes of acid rain. They get mixed with the rain and fall on the earth’s surface as acid rain. They may also precipitate in other forms such as sleet, snow, hail, or even fog.
Why does acid rain occur?
We know that it is caused due to a high level of pollution in the atmosphere. However, the actual chemistry of how acid rain precipitates onto the Earth is slightly more complicated. Let us take a detailed look at the chemistry and causes of acid rain.
How do pollutants get into the atmosphere?
These pollutants are transported into the atmosphere by wind and air currents. Here they react with the water in the atmosphere and oxygen in the air to form Sulphuric Acid and Nitric Acid respectively. These acids then lower the pH value of rainwater, making it acidic and harmful.
What is the pH of rainwater?
The pH level of rainwater is supposed to be 5.6. While the pH value of water is 7 which is what we consider neutral, rainwater has a more acidic nature. This is because there are some acidic compounds that are naturally mixed in with rainwater. These compounds, such as Nitrous oxide, carbon dioxide etc, are found in the lower levels ...
What is the biggest cause of sulfur dioxide?
The biggest cause is electricity production by burning coal. This thermal electricity plants are responsible for about 70% of Sulphur Dioxide release in the world. They also release huge quantities of NO about 20% in total. Any type of fossil fuels that we burn release both these pollutants.
Where do pollutants deposit?
They may also deposit on the earth’s surface in a dry form. This happens more in an area that receives an infrequent and low amount of rainfall, like deserts and arid areas. Dry deposition occurs when the pollutants form particles in the air. These particles settle on the earth’s surface on land and lakes, ponds etc.
Is acid rain toxic to humans?
Acid Rain is highly toxic to all living organisms in the world, and a major cause of land and soil pollution.
What is the effect of acid rain on soil?
In acid-sensitive areas, acid rain also depletes soil of important plant nutrients and buffers, such as calcium and magnesium, and can release aluminum, bound to soil particles and rock, in its toxic dissolved form. Acid rain contributes to the corrosion of surfaces exposed to air pollution and is responsible for the deterioration ...
What is the pH of acid rain?
acid rain, also called acid precipitation or acid deposition, precipitation possessing a pH of about 5.2 or below primarily produced from the emission of sulfur dioxide (SO 2) and nitrogen oxides (NO x; the combination of NO and NO 2) from human activities, mostly the combustion of fossil fuels.
How does acid deposition affect biodiversity?
In acid-sensitive landscapes, acid deposition can reduce the pH of surface waters and lower biodiversity. It weakens trees and increases their susceptibility to damage from other stressors, such as drought, extreme cold, and pests.
When was acid rain invented?
The phrase acid rain was first used in 1852 by Scottish chemist Robert Angus Smith during his investigation of rainwater chemistry near industrial cities in England and Scotland. The phenomenon became an important part of his book Air and Rain: The Beginnings of a Chemical Climatology (1872).
Is acid rain a global issue?
As a global environmental issue, it is frequently overshadowed by climate change. Although the problem of acid rain has been significantly reduced in some areas, it remains an important environmental issue within and downwind from major industrial and industrial agricultural regions worldwide. Britannica Explores.