
...
| Difference between Atom and Ion | |
|---|---|
| Unstable | Stable |
What two particles belong to the same element?
Any two atoms that have the same number of protons belong to the same chemical element. But atoms with an equal number of protons can have a different number of neutrons, which are defined as being different isotopes of the same element.
Which of the following is the same for an atom and its ion?
Solution : nuclear charge of an atom and is corresponding ion is same.
Which two atoms are of the same element?
Isotopes are atoms with different atomic masses which have the same atomic number. The atoms of different isotopes are atoms of the same chemical element; they differ in the number of neutrons in the nucleus.
Which 2 particles in an atom have the same charge?
The electrons have a negative electrical charge. An atom usually contains an equal number of positively charged protons and negatively charged electrons.
What is an example of an atom and an ion?
A neutral sodium atom, for example, contains 11 protons and 11 electrons. By removing an electron from this atom we get a positively charged Na+ ion that has a net charge of +1. Atoms that gain extra electrons become negatively charged. A neutral chlorine atom, for example, contains 17 protons and 17 electrons.
What is an atom and ion?
Atoms are single neutral particles. Molecules are neutral particles made of two or more atoms bonded together. An ion is a positively or negatively charged particle.
What are atoms of the same element called?
Atoms of the same element (i.e., same atomic number, Z) that have different numbers of neutrons are called isotopes. For example, 99% of the carbon atoms on Earth have 6 neutrons and 6 protons in their nuclei; about 1% of the carbon atoms have 7 neutrons in their nuclei.
Which of the following are the same for atoms of the same element?
Atoms of the same element have the same atomic number so the number of protons (and electrons) are same. But they have same or different mass numbers (like that for isotopes). Different mass number will have different number of neutrons. Was this answer helpful?
What is the name of atoms of the same element?
Isotopes are atoms of the same element (same number of protons) that have different numbers of neutrons in their atomic nuclei.
Which are the two charged particles of an ion?
ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Positively charged ions are called cations; negatively charged ions, anions.
What are ions that have the same charge?
In many cases, elements that belong to the same group (vertical column) on the periodic table form ions with the same charge because they have the same number of valence electrons. Thus, the periodic table becomes a tool for remembering the charges on many ions.
When two of the same type of atoms bond together this is a?
A Nonpolar Covalent Bond is created when atoms share their electrons equally. This usually occurs when two atoms have similar or the same electron affinity.
Which of the following is the same for an atom of the same elements?
Atoms of the same element have the same atomic number so the number of protons (and electrons) are same.
What are the similarities and differences between atoms and ions?
Basic difference between an atom and an ionATOMIONAtoms together generate moleculesAn electrovalent bond is formed between ionsThe number of electrons and protons is the sameElectrons and protons are unequal in number and hence the ions are unstable.2 more rows
What is the same about ions?
3:354:22Isotopes vs Ions | What is the Difference? | - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd an ion has the same number of protons.MoreAnd an ion has the same number of protons.
Which of the following refers to atoms of the same?
Atoms of the same element having the same atomic number but different mass are called isotopes. They have same number of protons and electrons but different number of neutrons.
How many electrons does a chlorine atom have?
Negative ions or anions are formed when an atom gains an electron. For example, a chlorine atom consists of 17 electrons and 17 protons. When it gains an electron, it becomes Cl⁻ ion. The charge of this ion is −1.
What happens when an atom loses an electron?
Unlike atoms, ions are charged particle. But they also consist of electrons, protons and neutrons. When an atom loses or gains an electron, it becomes an ion. Depending upon the gain or lose, atoms can be classified as positive ions or cations and negative ions or anions
How many protons does sodium have?
Positive ions or cations are formed when an atom loses an electron. This concept can be cleared with the help of an example. A sodium atom consists of 11 protons and 11 electrons. It is electrically neutral when it loses an electron it becomes Na⁺ ion. The charge of this ion is +1
How many neutrons are in oxygen?
Number of neutrons in oxygen is 16–8 = 8. v. The arrangement of the atoms in the periodic table is in such a way that down the group, the radius of the atom increases and across the period radius of the atom decreases.
Which model did not explain the fine structure of spectral lines of hydrogen?
Bohr atom model could not explain some concepts like the fine structure of spectral lines of hydrogen, distribution of electrons etc. Somerfield modified Bohr atom model and he explained the path of the electron and gave more explanations regarding the structure of spectral lines of hydrogen.
How are molecules formed?
Molecules are formed by the combination of atoms. iv. On the basis of nuclei, atoms can be classified as isotopes, isobars and isotones. a. Isotopes. If the atoms of the same element have the same atomic number, (represented as ‘Z’) and different mass number (represented as A). They are known as isotopes.
What is the name of the theory that states that atoms are the building blocks of matter?
It was known as Dalton’s atomic theory. According to this theory, the atoms are the building block of matter. But this clarification of atoms doesn’t exist for a long time and in 1815 another scientist name Prout suggested that elements are made up of hydrogen atoms.
What are the three particles in an atom?
The answers are as follows: 1. C. An atom is made up of three particles, which are electron, proton and neutron. The proton and the neutron are located in the central part of the atom, which is called the nucleus while the electrons orbit around the nucleus.
What is the difference between isotopes?
An isotope is an element which exist in several forms. The major difference between isotopes is the number of neutrons in their nucleus and this neutron number eventually determine their relative atomic mass. Thus two isotopes will have different neutron number and different mass number. 6.
What does a scientist use to study the particles inside the protons of a helium atom?
8. A scientist uses an accelerator and high energy electrons to study the particles inside the protons of a helium atom. What particles is the scientist studying?
What is the particle that orbits the nucleus called?
A particle that orbit the nucleus is called AN ELECTRON. An electron is the negatively charged component of the atom, which orbit round the nucleus of an atom. The number of electrons that are orbiting round the nucleus of a particular atom depends on the atomic number of that atom. 4.
What element has four protons and five neutrons?
The proton number of four indicates that the element we are searching for is beryllium, because beryllium has four protons and five neutrons in its nucleus. 7. B. The atomic number of iron is 26 but its mass number is 56.
Where are quarks found in an atom?
Quarks are sub-atomic particles which are usually find inside the proton an the neutron which are located in the nucleus of an atom. High energy electrons and accelerators are usually used to study this sub particles. The electrons which orbit round the nucleus do not have quarks in their interiors.
Where are the electrons and neutrons in sulfur?
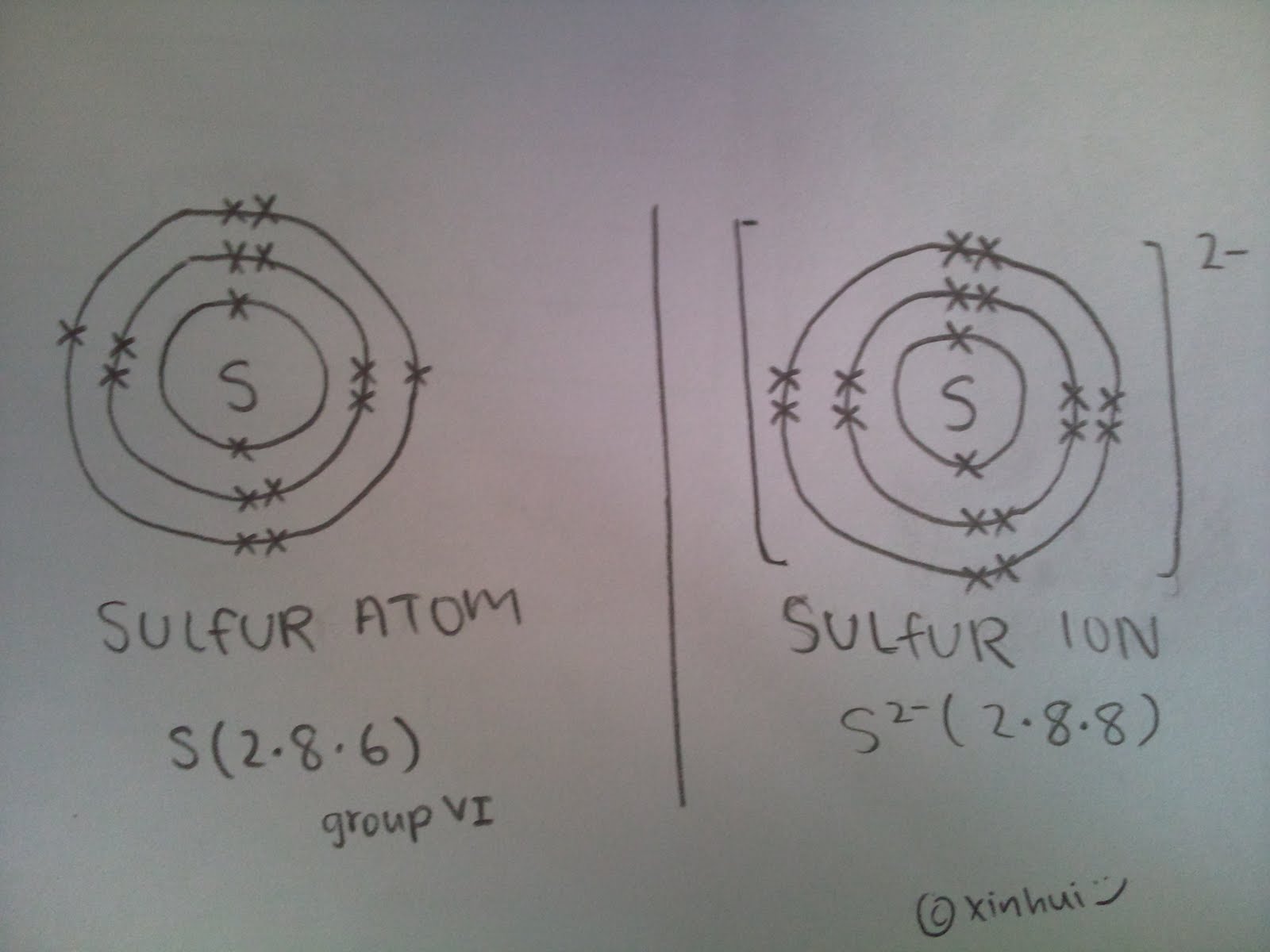
They are usually find in the proton and the neutron which are located inside the nucleus of an atom. Quark is not found in electrons. 9. D. The atomic number of sulfur is 16, that means that, it has 16 electrons, 16 protons and 16 neutrons in its atom at the neutral state.
What is the nucleus of an atom?
The nucleus of an atom contains neutrons and protons bonded tightly together. The same chemical element can have a different number of neutrons and still be the same element. We refer to the atoms of the same element with different numbers of neutrons as "isotopes".
What atom reacts with water more violently than an ionized atom?
For instance, a neutral sodium atom (say, from a chunk of sodium metal) reacts with water much more violently than an ionized sodium atom (say, from a bit of salt). Chemists know this very well. It's not enough to say what atoms are involved if you want to fully describe and predict a reaction.
How do two atoms of the same element and in the same electronic state affect their ability to chemically bond?
Two atoms of the same element and in the same electronic state could be traveling or rotating at different speeds, which affects their ability to chemically bond. Slower moving atoms (such as the atoms in solid iron) have time to form stable bonds, while faster moving atoms (such as the atoms in liquid iron) cannot form such stable bonds.
What happens when copper atoms are excited?
The excited copper atom will emit a bit of light when the electron relaxes back down to the ground state , and the copper atom already in the ground state will not.
What is the most common nuclear reaction?
The most common nuclear reaction on earth is radioactive decay. Some isotopes decay very quickly into other elements and emit radiation, while other isotopes do not. If you are doing carbon dating, the fact that a carbon-12 atom is not identical to a carbon-14 atom is essential to the dating process.
Can gold atoms have different excited states?
No. Just like the electrons, the neutrons and protons in the nucleus can be in various excited states. In addition, the nucleus as a whole can rotate and vibrate at various speeds. Therefore, even if all else is identical, two gold atoms can have their nuclei in different excited states and behave differently in nuclear reactions.
Can two atoms be chemically identical?
No. Although two such atoms are essentially chemicallyidentical (they will chemically react in the same way), they are not completely identical. There's more to the atom than the electrons. There's also the nucleus. The nucleus of an atom contains neutrons and protons bonded tightly together. The same chemical element can have a different number ...
What is the simplest atom of an element?
The simplest atom of an element is a substance called “hydrogen” (it is a clear colorless gas that is part of out atmosphere). Hydrogen atoms are made from just one proton and one electron. There are usually no neutrons in a hydrogen atom. In fact, there are special names for hydrogen atoms that do have neutrons in them (atoms with different numbers of neutrons, but always the same number of protons are called “isotopes”. An isotope can happen for any element that has more than one form, and more than one “weight” but still the same number of protons.) In the case of hydrogen, when one neutron is added it is called “deuterium” and if it has two neurons, it is called “tritium”. Notice that “tritium” starts with the same first letters “tri” as the spelling of “tricycle”. A tricycle has 3 wheels. A tritium atom has 2 neutrons, plus one proton, so it has a “weight” of 3. The weight is different, but it still has only one proton, so it is still a form of hydrogen.
What are particles in physics?
There really are no tiny “particles.” Simple fundamental quantum objects such as electrons, photons, quarks, and neutrinos are often called “particles” but they should be called “quanta.” They are spatially-extended highly-unified objects that obey the rules of quantum physics. Each one in principle occupies all space, but in practice is only likely to “be found” over a relatively small (but non-zero) volume of space. Atoms also merit the name “quanta,” but atoms are composite quantum objects comprising many fundamental quanta: several electrons, several protons and neutrons (each of them comprising three quarks), and occasional photons that are absorbed or emitted by the atom. So “particles” (i.e. simple fundamental quanta) and atoms (i.e. composite quanta) are somewhat different, even though both fundamental quanta and atoms obey the quantum rules. For more details, see:
What is an element called?
An “atom” is the smallest “piece” of something that can exist without breaking it down into parts. An atom’s parts are called “protons”, “electrons” and in most cases some number of heavy but neutral particles that are very much like protons except that protons have what we call a “positive electrical charge” while neutrons have no charge. Protons and their “positive electrical charge” are usually balanced by an equal number of electrons with a “negative electrical charge”, in fact, the smallest possible amount of electrical power is called “one electron volt”.
What is the atomic number of an element?
An element has a specific atomic number which is the number of protons in the nucleus of the atom. This is usually balanced by an identical number of electrons (which determine the chemical behavior of the element).
What is the atomic mass of sulfur?
So we must calculate a bit: The Atomic mass (in atomic mass units) of the proton is 1.007276 .
Why do most elements have more than one isotope?
But most elements (all elements if we include radio-active ones) usually have more than one isotope because the number of neutrons (and therefore mass number) can vary. Mass, in contrast to charge, has only minor effects on chemistry.
What are the elements that have been added to the list of known elements since World War II?
Physicists have officially added 26 new elements since World War II to the list of known ones, with names like americium, plutonium, einsteinium, nobelium, fermium, flerovium and organessen. The latter is the latest discovery and the heaviest, being a noble gas like neon and argon, but so heavy as to likely be a solid at normal temperatures. It’s hard to say because there have only been, perhaps, a few hundred atoms created, and they decay within less than a second of their creation.
Introduction to Atoms
- The word ‘atom’ was coined from the Greek word ‘atomos’; the meaning of ‘a’ in the word is ‘not’ and ‘tomos’ means ‘cut’. Together it became impossible to cut. Investigations on atoms have been started over a long period of time and still, it is continuing. The basic definition of atoms is “Atoms are small indivisible particles and every matter tha...
Features of Atom on The Basis of Modern Atomic Theory
- The Modern atomic theory states that though atoms are small, they are divisible.
- Nucleus of an atom consists of subatomic particles, protons and neutrons which are collectively known as nucleons. Electrons are revolving around the nucleus in specific orbits. Since the number of...
- Molecules are formed by the combination of atoms.
- The Modern atomic theory states that though atoms are small, they are divisible.
- Nucleus of an atom consists of subatomic particles, protons and neutrons which are collectively known as nucleons. Electrons are revolving around the nucleus in specific orbits. Since the number of...
- Molecules are formed by the combination of atoms.
- The arrangement of the atoms in the periodic tableis in such a way that down the group, the radius of the atom increases and across the period radius of the atom decreases.
Ions
- Unlike atoms, ions are charged particles. But they also consist of electrons, protons and neutrons. When an atom loses or gains an electron, it becomes an ion. Depending upon the gain or loss, atoms can be classified as positive ions or cations and negative ions or anions. 1. Positive Ions (Cations) Positive ions or cations are formed when an atom loses an electron. This concept can …
Difference Between Atoms and Ions
- Atoms are electrically neutral, which means the number of protons and electrons will be the same. While ions are charged particles, positive ions (cations) or negative ions (anions).
- Combination of atoms results in the formation of molecules and the combination of ions results in the formation of compounds.
- Independent existence of atoms is not possible. But, ions can exist independently.
- Atoms are electrically neutral, which means the number of protons and electrons will be the same. While ions are charged particles, positive ions (cations) or negative ions (anions).
- Combination of atoms results in the formation of molecules and the combination of ions results in the formation of compounds.
- Independent existence of atoms is not possible. But, ions can exist independently.
- Ions are stable whereas atoms are unstable.