
Protons and neutrons have approximately the same mass, about 1.67 × 10-24 grams, which scientists define as one atomic
Nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission bomb) or a combination of fission and fusion (thermonuclear weapon). Both reactions release vast quantities of energy from relatively small amounts of matter.
Atomic mass unit
The unified atomic mass unit (symbol: u) or dalton (symbol: Da) is the standard unit that is used for indicating mass on an atomic or molecular scale (atomic mass). One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.
John Dalton
John Dalton FRS was an English chemist, physicist, and meteorologist. He is best known for introducing the atomic theory into chemistry, and for his research into color blindness, sometimes referred to as Daltonism in his honour.
What two particles have approximately the same mass?
What two particles have approximately the same mass? The nucular particles: protons and neutrons. The nuclear particles, protons and neutrons, are conceived to have a positive electronic charge, and a neutral charge respectively. The protonic mass is 1.672621898(21) × 10−27 ⋅ kg.
What is the mass of a proton and a neutron?
The nuclear particles, protons and neutrons, are conceived to have a positive electronic charge, and a neutral charge respectively. The protonic mass is #1.672621898(21)xx10^(−27)*kg#. And the neutron mass is #1.674927471(21)xx10^(−27)* kg#, so to a first approx. these masses are equal.
What is the charge of protons and neutrons?
The nucular particles: protons and neutrons. The nuclear particles, protons and neutrons, are conceived to have a positive electronic charge, and a neutral charge respectively. The protonic mass is 1.672621898(21) × 10−27 ⋅ kg.
What is the force of repulsion between protons and neutrons?
Data are from this site, and this site. Protons and neutrons engage in the strong nuclear force, which, at nuclear ranges, is stronger than the electrostatic forces of repulsion that operates between the protons.
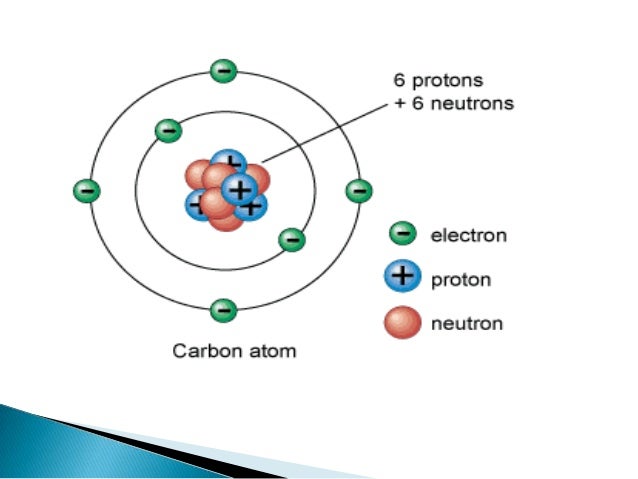
Which two particles in an atom have the same mass?
Protons and neutrons have approximately the same mass. However, one proton is about 1,835 times more massive than an electron. Atoms always have an equal number of protons and electrons, and the number of protons and neutrons is usually the same as well.
What two particles are equal in an atom?
Protons and electrons are present in equal numbers in neutral atoms.
What are two particles?
There are three subatomic particles: protons, neutrons and electrons.
Which particles are equal in number in a neutral atom?
Fundamental Subatomic Particles The number of electrons in a neutral atom is equal to the number of protons. The mass number of the atom (M) is equal to the sum of the number of protons and neutrons in the nucleus.