What type of change is freezing water?
physicalWhen liquid water (H2O) freezes into a solid state (ice), it appears changed; however, this change is only physical, as the composition of the constituent molecules is the same: 11.19% hydrogen and 88.81% oxygen by mass. (Public Domain; Moussa). Physical changes can further be classified as reversible or irreversible.
Is freezing a chemical change or physical change?
physicalResearch shows that students frequently use the term chemical change to describe changes in physical state. Freezing and boiling are considered to be examples of chemical reactions.
Is freezing of water a physical change?
Freezing of water is a chemical change. Freezing of water is a chemical change.
What is freezing water an example of?
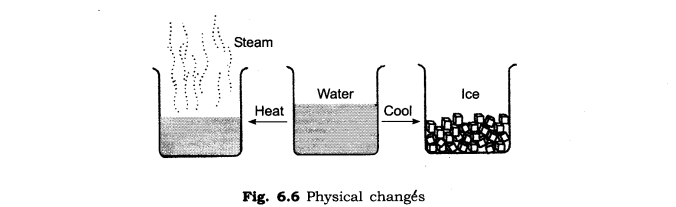
Freezing water is an example of a phase transition -- a change in the physical properties of a substance when the temperature or pressure are changed.
Is freezing of water a chemical process?
Freezing is not a chemical reaction, but is a phase (or physical) change.
Why is freezing a chemical change?
Freezing is a physical change. It involves a liquid changing states to a solid. Thus, freezing a substance will not change its chemical identity, but its state.
Why freezing of water is a reversible change?
Solution: (a) Reversible changes are changes in a substance that can be reversed back to their original state. Irreversible changes are changes in substance that will have a permanent effect, and they cannot be reversed. Freezing of water is a reversible change because frozen water can be melted on heating.
Is freezing a physical property?
Physical properties are characteristics of a substance. They do not change. Physical properties include color, smell, freezing/melting point, and density.
Why is freezing water not a chemical change?
If liquid water is boiled, it is still water; likewise frozen water, or ice, is still water. Melting, boiling, or freezing simply by the application of a change in temperature are examples of physical changes, because they do not affect the internal composition of the item or items involved.
Is the freezing of water an endothermic or exothermic process explain?
When water becomes a solid, it releases heat, warming up its surroundings. This makes freezing an exothermic reaction.
Which type of changes are melting freezing and boiling?
A phase change is a physical process in which a substance goes from one phase to another. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or the boiling point of the substance.
Is freezing kinetic or potential energy?
As water freezes, it does so because after thermal energy leaves the particles (freezing is exothermic), the average translational+rotational kinetic energy decreases, but the total average kinetic energy does not... and the temperature does not change either.
Why is freezing called a physical change?
Question: Why is freezing of water considered as a physical change? Answer: This is because all you are doing is changing the physical state of water from a liquid to solid but it doesnot alters it's chemical properties.
Is freezing a physical property?
Physical properties are characteristics of a substance. They do not change. Physical properties include color, smell, freezing/melting point, and density.
Why is freezing water not a chemical change?
If liquid water is boiled, it is still water; likewise frozen water, or ice, is still water. Melting, boiling, or freezing simply by the application of a change in temperature are examples of physical changes, because they do not affect the internal composition of the item or items involved.
Is ice melting a chemical change?
The melting of ice is a physical change when it occurs naturally. But when you speed up the process by using a reactant, such as salt, it becomes a chemical reaction.
What Happens When Water Freezes
When water freezes into ice, some of its properties change, but the essence of water remains the same. Before and after the change, the same number of water molecules are present, and the chemical properties of water remain constant. However, to form water, hydrogen and oxygen atoms must undergo chemical transformations.
Light Provides Energy
However, light energy does not produce the atoms that make up these sugars—which would violate the law of conservation of mass—it simply provides energy for chemical change. When water turns into a solid or gas, we say that it goes into another state of matter. Although the physical form of water changes, its molecules remain the same.
The Physics of Boiling Water
Boiling water is an example of physical and non-chemical change because water vapor still has the same molecular structure as liquid water (H2O). Since the actual molecular structure of water does not change and there is no chemical reaction involving water molecules, the transition is a physical change.
What Happens When Ice Melts
When heat (a form of energy) is added, the ice melts and turns into liquid water. The removal of heat causes water (liquid) to freeze, forming ice (solid). When water becomes solid, it releases heat, warming the environment.
The Conditions of Saltwater
For example, the dissolution of salt in water is usually considered a physical change, however the chemicals in saline (sodium hydrate and chloride ions) are different from those in solid salt.
State Changes Are Not Chemical Changes
As water evaporates, the chemical formula of water will also be the same. If liquid water turns into ice, then it undergoes physical rather than chemical changes. In the case of water freezing, the water particles did not change their composition.
Adding Particles to Water Lowers Its Freezing Point
If you add other substances to the water, such as sugar or salt, the temperature will drop below 32 degrees before ice starts to form. The new freezing point depends on what’s added and how it mixes with water, which is why cities in some states sprinkle roads with salt in winter to remove snow and ice.
What Chemical Properties Are
Chemical properties are properties that can only be measured or observed when a substance changes, becoming a completely different type of substance. Properties such as size, shape, color and state of matter are called physical properties.
What State Changes Are
Changes in state can, of course, be purely physical, such as when liquid water is boiled to form steam. Water, like all other types of matter, requires the addition or removal of energy in order to change state. Freezing or solidification is the removal of heat from a substance to change that substance from a liquid to a solid.