When was the atom first discovered?
The first truly direct evidence of atoms is credited to Robert Brown, a Scottish botanist. In 1827, he noticed that tiny pollen grains suspended in still water moved about in complex paths. This can be observed with a microscope for any small particles in a fluid.
How did they discover the atom?
How Did Democritus Discover the Atom? Because ancient Greek thinkers such as Democritus lacked sophisticated technology and tools such as the microscope, his theory of the atom was due more to thought experimentation than to hard empirical observation, as used in modern science. In essence, he conceptualized it.
Why was the Atom an important discovery?
Why Are Atoms Important? Atoms are important because they form the basic building blocks of all visible matter in the universe. There are 92 types of atoms that exist in nature, and other types of atoms can be made in the lab. The different types of atoms are called elements.
When did Democritus make his discovery about the atom?
Greek philosophers Leucippus and Democritus first developed the concept of the atom in the 5th century B.C.E. However, since Aristotle and other prominent thinkers of the time strongly opposed their idea of the atom, their theory was overlooked and essentially buried until the 16th and 17th centuries.
.PNG)
Who really discovered atoms?
The first modern evidence for atoms appears in the early 1800s when British chemist John Dalton discovered that chemicals always contain whole number ratios of atoms.
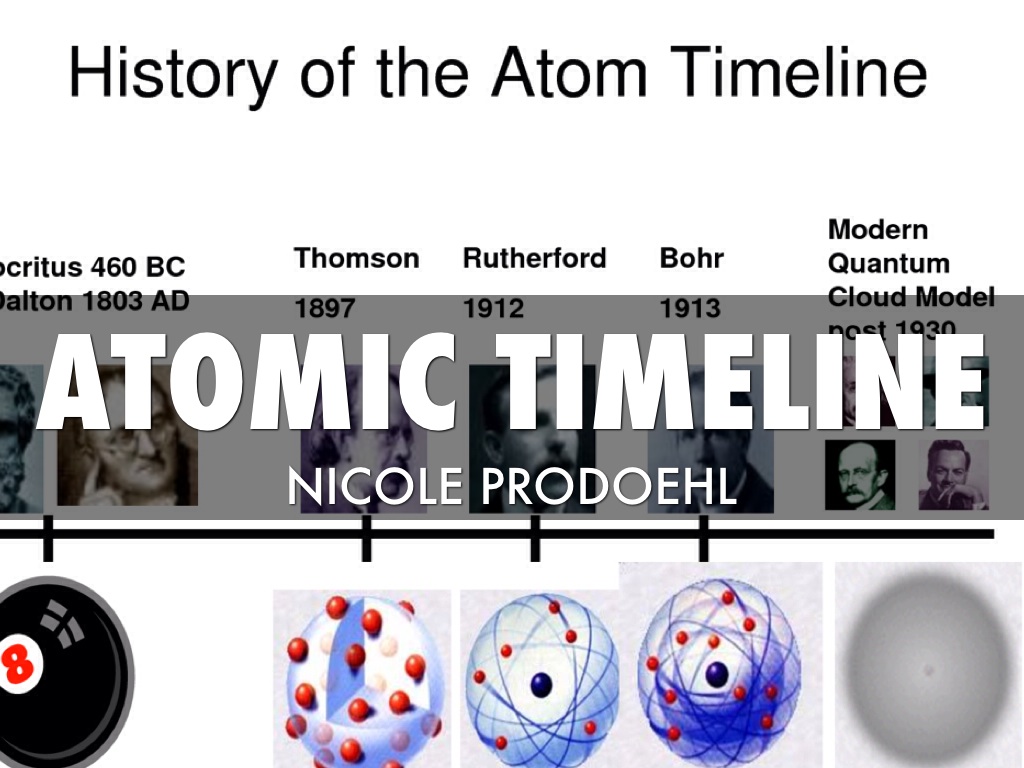
What is an atomic timeline?
The Atomic Timeline follows man's discovery and research of atoms, more widely Chemistry. It dates back to 440 BCE, when Democritus discovered the atom. The timeline covers our discoveries, who made them, and enables us to compare those discoveries made long ago to what we know today about the intriguing atom.
Which model of the atom came first?
Dalton's model (1803)
When did they discover atoms?
Around 450 B.C., the Greek philosopher Democritus introduced the idea of the atom. However, the idea was essentially forgotten for more than 2000 years. In 1800, John Dalton re-introduced the atom. He provided evidence for atoms and developed atomic theory.
What are the atomic theories in order?
List of Atomic TheoriesAncient Greek Beliefs.Dalton's Atomic Theory.J.J. Thomson's Theory.Rutherford's Hypothesis.Bohr's Theory.Einstein, Heisenberg and Quantum Mechanics.
What is J.J. Thomson atomic theory?
J.J. Thomson's experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. Thomson proposed the plum pudding model of the atom, which had negatively-charged electrons embedded within a positively-charged "soup."
What was John Dalton's atomic theory?
The first part of his theory states that all matter is made of atoms, which are indivisible. The second part of the theory says all atoms of a given element are identical in mass and properties. The third part says compounds are combinations of two or more different types of atoms.
What was Aristotle's atomic theory?
2.1 Atomism in Aristotle and Boyle. In Aristotle's time, atomists held that matter was fundamentally constructed out of atoms. These atoms were indivisible and uniform, of various sizes and shapes, and capable only of change in respect of position and motion, but not intrinsic qualities.
Who created the first successful model of an atom?
Niels Bohr created the first successful model of an atom. The model showed that the electrons orbit the nucleus just like the planets orbit the sun. This is incorrect however, considering that it is impossible to know exactly how they are actually moving.
What did Thompson conclude about atoms?
Thompson then concluded that atoms must contain negatively charged particles; discovering the electron . He also made the Plum Pudding model of an atom where the electrons were evenly spread throughout a sphere of positvely charged material. http://www.nobelprize.org/nobel_prizes/physics/laureates/1906/thomson.jpg.
How did James Chadwick prove the existence of neutrons?
James Chadwick proved the existance of neutrons by bombarding beryllium with alpha particles. While doing this, he noticed a particle with a mass very close to that of a proton being released. Considering the fact that they were not bent by electrical fields and were highly penetrating, these particles were electrically neutral.#N#http://web.me.com/dtrapp/people2.f/Chadwick.jpg
Which atom has a very small mass?
Neutron. Ernest Rutherford showed that the atom was a lot of empty space, with a small positively charged nucleus that contained most of the mass, and a very small mass of negatively charged particles orbiting the nucleus.
What did Dalton discover about atoms?
Dalton realized that if elements were made up of atoms. And a combination of atoms are called a molecule. Dalton also came to the theory that atoms are indestructible and can not be cut anymore. From this point on, atomic theory is based off his findings. Feb 1, 1896.
Who discovered that all atoms have electrons outside the nucleus?
J.J. Thomson discovered that all atoms have electrons outside the nucleus. He discovered this by doing a series of experiements desgined to study the nature of electric discharge in a high-vacuum cathode-ray tube. Thomson electrically charged plates and magnets as evidence of "bodies smaller than regular atoms.".
What is Max Planck's theory?
Max Planck's Atomic Theory. Max Planck developed the quantum theory which was based off of the fact that he believed that all elements eventually lost their heat or energy over time. So he came up with the equation to figure out the energy of the atom or elements; h=6.63*10E-34 Js.
When did Democritus discover the atomic theory?
Democritus discovers his atomic theory about atoms around 400 B.C.E. His theory states that everything around us is made up of atoms. He also stated that atoms are indivisble and cannot be broken down. Finally, he said that in between the atoms, there is a bunch of free space and the morfe empty space there is, the heavier the atom gets. Democritus help influential help from Leucippus, his mentor. (Couild go to B.C.E and the earliest date i could get was 100 A.D.)
What did Rutherford discover about the atom?
Rutherford published his atomic theory discribing the atom as having a central nucleus (positive) surrounded by negative orbiting electrons. He found this out by doing an experiment with his gold foil model which contained a detecting screen (acting as the membrane), particle emitter, and silt.
What did Marie and Pierre Curies discover?
Marie & Pierre Curies' Discovery. The Curies are credited with discovering radium and polonium when they started to investigate radioactive substances. She found these two and many more elements from the element, uranium and it contained some traces of radioactivity.
Which theory did Niels Bohr apply to Rutherford's atomic structure?
Niels Bohr's Atomic Theory and Model. The Bohr Model Bohr applied the quantum theory to Rutherford's atomic structure by assuming that all electrons travel in stationary orbits defined by their momentum. This led to possible energy levels for their orbits.
Who was the first person to believe in the atom?
John Dalton. Born: September 6, 1766, Died: July 27, 1844. Democritus first suggested the existence of the atom but it took almost two millennia before the atom was placed on a solid foothold as a fundamental chemical object by John Dalton (1766-1844). Although two centuries old, Dalton's atomic theory remains valid in modern chemical thought.
Who created the atom model?
Erwin Schrodinger. A powerful model of the atom was developed by Erwin Schrödinger in 1926. Schrödinger combined the equations for the behavior of waves with the de Broglie equation to generate a mathematical model for the distribution of electrons in an atom.
What did Rutherford discover?
He discovered alpha and beta rays, set forth the laws of radioactive decay, and identified alpha particles as helium nuclei.
What did Democritus believe about atoms?
In addition, Democritus believed that the atoms differed in size and shape, were in constant motion in a void, collided with each other; and during these collisions, could rebound or stick together.
Who discovered the unit charge of an electron?
Robert Millikan (1868-1953) determined the unit charge of the electron in 1909 with his oil drop experiment at the University of Chicago. Thus allowing for the calculation of the mass of the electron and the positively charged atoms.
Who rejected Democritus' theory?
Although Democritus' theory was remarkable, it was rejected by Aristotle, one of the most influential philosophers of Ancient Greece; and the atomic theory was ignored for nearly 2,000 years.
Who believed that all substances on Earth are made of small particles called atoms?
Most people followed Aristotle’s idea, causing Democritus’ idea- which was that all substances on Earth where made of small particles called atoms- to be over looked for about 2,000 years! Aristotle's view was finally proven incorrect and his teachings are not present in the modern view of the atom.
Atomic Theory Definition
All matter in the universe is made up of atoms. For centuries, scientists have been fine-tuning the theory of the atom through experimentation and research. As a result of this research, many ideas have been pooled together in order to collaboratively agree on what an atom is thought to be today.
Atomic Theory Timeline
Like most theories, ideas about the atom have changed over time. What makes the theory of the atom slightly difficult to comprehend, is that an atom cannot be seen with the naked human eye. As a result, all theories rely on experimentation and assumptions.
Significance of Atomic Theory in Real Life
The importance of transitioning beliefs about the atomic structure can be easily overlooked. In reality, the process that the atomic theory has gone through is the epitome of what science is all about. Being able to build upon past theorists' works has made science boom into what it is today.
Who was the scientist who came up with the idea of the planetary model of the atom?
Niels Bohr agreed with the planetary model of the atom, but also knew that it had a few flaws. Using his knowledge of energy and quantum physics he was able to perfect Rutherford’s model. He was able to answer why the electrons did not collapse into the nucleus.
What is the negative charge of an atom called?
He then found out that this charge was 1000 times lighter that a hydrogen atom. He made a bold statement saying that this negative charge must be inside an atom. This negative charge (he called corpuscles) later became known as the electron.