Who discovered Boyle's Law?
The law was named after chemist and physicist Robert Boyle, who published the original law in 1662. This relationship between pressure and volume was first noted by Richard Towneley and Henry Power in the 17th century. Robert Boyle confirmed their discovery through experiments and published the results.
What is Boyle's Law in simple words?
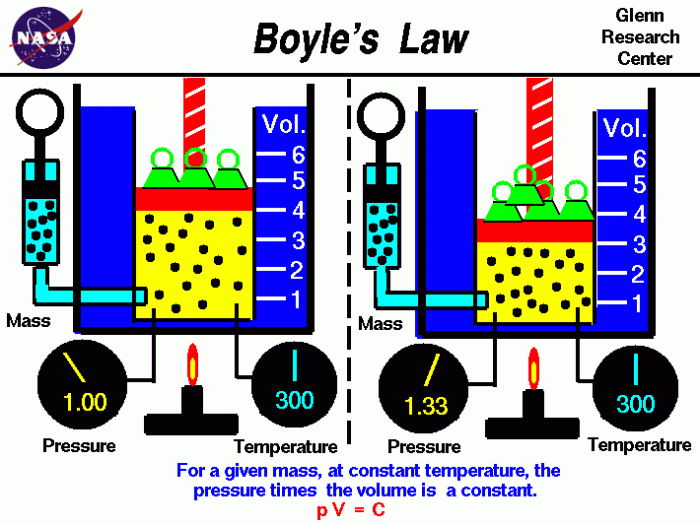
Definition. The law itself can be stated as follows: For a fixed amount of an ideal gas kept at a fixed temperature, pressure and volume are inversely proportional. Or Boyle's law is a gas law, stating that the pressure and volume of a gas have an inverse relationship, when temperature is held constant.
How does Boyle's Law relate to kinetic and ideal gases?
Relation with kinetic theory and ideal gases. Boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its pressure.
Is Boyle’s law related to Boyle-Mariotte law?
Clearly the two lines of reasoning are tightly interrelated. Boyle’s law is sometimes known as the Boyle-Mariotte law, to acknowledge the 1676 contributions of the French physicist Edme Mariotte. There is some question as to whether Mariotte did in fact discover the principle independently of Boyle’s writings.

When was Boyle's law invented?
1662The law, discovered by Robert A. Boyle in 1662, states that at a fixed temperature, the volume of gas is inversely proportional to the pressure exerted by the gas.
What is Robert Boyle's law?
Boyle observed that the product of the pressure and volume are observed to be nearly constant. The product of pressure and volume is exactly a constant for an ideal gas. p * V = constant. This relationship between pressure and volume is called Boyle's Law in his honor.
What was Robert Boyle's experiment?
Boyle's most famous experiments with gases dealt with what he called the "spring of air." These experiments were based on the observation that gases are elastic. (They return to their original size and shape after being stretched or squeezed.)
How was Boyle's law discovered?
Boyle discovered the relationship between pressure and volume in a gas that is now known as Boyle's law by using a vacuum chamber to change the pressure in a gas. He made careful observations and conducted a series of experiments to show that pressure and volume are inversely proportional to each other.
Why is Boyle's law important?
Why is Boyle law important? Boyle's law is significant because it explains how gases behave. It proves beyond a shadow of a doubt that gas pressure and volume are inversely proportional. When you apply pressure on a gas, the volume shrinks and the pressure rises.
What did Robert Boyle say about God?
God, he believes, could have started things off earlier or later, but chose not to. Having created matter, he broke it up and started it moving. Sometimes Boyle says he broke it up by starting it moving.
What was Boyle famous quote?
“Those distinct substances, which concretes generally either afford, or are made up of, may, without very much inconvenience, be called the elements or principles of them.”
How do we use Boyle's law in everyday life?
You can observe a real-life application of Boyle's Law when you fill your bike tires with air. When you pump air into a tire, the gas molecules inside the tire get compressed and packed closer together. This increases the pressure of the gas, and it starts to push against the walls of the tire.
Which statement best describes Boyle's law?
The temperature of fixed mass of gas at a constant pressure is inversely proportional to its volume.
How do you use Boyle's law?
Let's say we change the volume of a gas under isothermal conditions, and we want to find the resulting pressure. Then, the equation of Boyle's law states that: p₂ = p₁ * V₁ / V₂ or p₂ / p₁ = V₁ / V₂ . As we can see, the ratio of the final and initial pressure is the inverse of the ratio for volumes.
What are the 6 gas laws?
Gas Law Formula TableGas LawFormulaBoyle's LawP1V1=P2V2Gay- Lussac LawP1/T1=P2/T2Avogadro's LawV / n = constantIdeal Gas LawPV=nRT1 more row
Which statement is correct with Boyle's law?
The correct option is A At a given temperature, pressure of a given mass of gas is inversely proportional to its volume.
What is the relationship between pressure and volume?
This empiricalrelation, formulated by the physicist Robert Boylein 1662, states that the pressure(p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; i.e., in equation form, pv= k, a constant. The relationship was also discovered by the French physicist Edme Mariotte(1676).
What is the name of the first gas law?
See Article History. Alternative Titles: Mariotte’s law, first gas law. Boyle ’s law, also called Mariotte’s law , a relation concerning the compression and expansion of a gas at constant temperature. This empirical relation, formulated by the physicist Robert Boyle in 1662, states that the pressure ...
What is an encyclopedia editor?
Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. ...
Who discovered the relationship between volume and pressure?
The relationship was also discovered by the French physicist Edme Mariotte (1676). Boyle's law, showing the relationship between volume and pressure when mass and temperature are held constant. The law can be derived from the kinetic theory of gases assuming a perfect (ideal) gas ( see perfect gas ).
Which law states that the pressure exerted by a given gas is proportional to its density?
Boyle’s law—that the pressure exerted by a given gas is proportional to its density if the temperature is kept constant as the gas is compressed or expanded—follows immediately from Bernoulli’s assumption that the mean speed of the molecules is determined by temperature alone. Departures from…
Who first described the elastic properties of gas?
The first is Boyle’s law, which refers to the elastic properties of the gas; it was described by the Anglo-Irish scientist Robert Boyle in 1662 in his famous “ . . . Experiments . . . Touching the Spring of the Air . . . .” It states…
Do real gases obey Boyle's law?
Real gases obey Boyle’s law at sufficiently low pressures, although the product pv generally decreases slightly at higher pressures, where the gas begins to depart from ideal behaviour. Demonstration of Boyle's law showing that for a given mass, at constant temperature, the pressure times the volume is a constant.
What did Boyle attempt to do?
Explaining all gases were made of tiny particles, Boyle attempted to build a universal ‘corpuscular theory’ of chemistry. He was able to give meaning to the concept of “elements” as well as giving us the litmus test.
What is the law that states that the pressure of a gas varies inversely with its volume?
The statement that “at constant temperature, the pressure of a gas varies inversely with its volume” has become known universally as Boyles Law , it appears in an appendix written in 1662 to his work New Experiments Physio-Mechanicall, Touching the Spring of the Air and its Effects in 1660.
What is the theory that a gas becomes less?
He is also credited with defining the theory known as “Boyle’s Law” for which he remains most famous. This states that if the volume of a gas becomes less, the pressure will increase proportionally. Explaining all gases were made of tiny particles, Boyle attempted to build a universal ‘corpuscular theory’ of chemistry.
What did the Sceptical Chymist write?
He covered many topics ranging from philosophy, medicine and religion, He also wrote The Sceptical Chymist in 1661, in which he attacked Aristotle’s theory of four elements. This was an essential part of the modern theory of chemical elements.
Who invented the vacuum chamber?
With Hooke’s mechanical talents acting as a foil to Boyle’s own researches into air (the properties of gases), they produced the vacuum chamber or air-pump. Boyle and Hooke were pioneers of their time.
Did Descartes believe in the vacuum?
Many scientists at the time did not believe that a vacuum could exist so Boyle was also constrained to provide a defense of his work with the vacuum within the 1662 appendix. Descartes for example believed in the existence of an “all-pervading ether” and although Boyle discounted this theory from a lack of scientific evidence he did agree that “the world was basically a complex system governed by a small number of simple mathematical laws”
Who was the Irish chemist who discovered that air has weight?
Robert Boyle will always be known as the Irish chemist who found out that air has weight and who was instrumental in defining chemical elements and chemical reactions as a clear separation from alchemy.
What did Boyle do in his will?
In his will, Boyle provided money for a series of lectures to defend the Christian religion against those he considered "notorious infidels, namely atheists, deists, pagans, Jews and Muslims", with the provision that controversies between Christians were not to be mentioned (see Boyle Lectures ).
How old was Boyle when he was sent to Eton College?
As a child, Boyle was fostered to a local family, as were his elder brothers. Boyle received private tutoring in Latin, Greek, and French and when he was eight years old, following the death of his mother, he was sent to Eton College in England.
What is Robert Boyle's law?
Boyle's law is named in his honour. The Royal Society of Chemistry issues a Robert Boyle Prize for Analytical Science, named in his honour. The Boyle Medal for Scientific Excellence in Ireland, inaugurated in 1899, is awarded jointly by the Royal Dublin Society and The Irish Times.
How many volumes of Robert Boyle's notebooks are there?
One of Robert Boyle's notebooks (1690-1691) held by the Royal Society of London. The Royal Society archives holds 46 volumes of philosophical, scientific and theological papers by Boyle and seven volumes of his correspondence.
What did Boyle say about beauty?
Boyle's writings mention that at his time, for "European Eyes", beauty was not measured so much in colour of skin, but in "stature, comely symmetry of the parts of the body, and good features in the face". Various members of the scientific community rejected his views and described them as "disturbing" or "amusing".
What was the merit of Boyle?
Boyle's great merit as a scientific investigator is that he carried out the principles which Francis Bacon espoused in the Novum Organum. Yet he would not avow himself a follower of Bacon, or indeed of any other teacher.
Where was Richard Boyle born?
Boyle was born at Lismore Castle, in County Waterford, Ireland, the seventh son and fourteenth child of The 1st Earl of Cork ('the Great Earl of Cork') and Catherine Fenton. Lord Cork, then known simply as Richard Boyle, had arrived in Dublin from England in 1588 during the Tudor plantations of Ireland and obtained an appointment as a deputy escheator. He had amassed enormous wealth and landholdings by the time Robert was born, and had been created Earl of Cork in October 1620. Catherine Fenton, Countess of Cork, was the daughter of Sir Geoffrey Fenton, the former Secretary of State for Ireland, who was born in Dublin in 1539, and Alice Weston, the daughter of Robert Weston, who was born in Lismore in 1541.
How does Boyle’s law work?
Or Boyle’s law is a gas law, stating that the pressure and volume of a gas have an inverse relationship. If volume increases, then pressure decreases and vice versa, when the temperature is held constant.
What are some examples of Boyle’s law in everyday life?
You can observe a real-life application of Boyle’s Law when you fill your bike tires with air. When you pump air into a tire, the gas molecules inside the tire get compressed and packed closer together. This increases the pressure of the gas, and it starts to push against the walls of the tire.
Where is Boyle’s law used?
Since the boiling point is dependent on pressure, you can use Boyle’s law and a syringe to make water boil at room temperature. Deep-sea fish die when they’re brought from the depths to the surface. The pressure decreases dramatically as they are raised, increasing the volume of gases in their blood and swim bladder.
Why Boyle’s Law is important?
According to Boyle’s law, if a given amount of gas has a constant temperature, increasing its volume decreases its pressure, and vice-versa. When you inhale, muscles increase the size of your thoracic (chest) cavity and expand your lungs.
How do you know when to use Boyle’s Law?
Since the volume of the gas is the only variable that has changed, we can use Boyle’s law in order to find the final pressure. Since pressure and volume are on the same side of the ideal gas law, they are inversely proportional to one another. In other words, as one increases, the other will decrease, and vice versa.
Does Boyle’s law have to be in ATM?
Example #10: A balloon contains 7.20 L of He. The pressure is reduced to 2.00 atm and the balloon expands to occupy a volume of 25.2 L.
Why temperature is constant in Boyle’s law?
How does temperature remain constant in Boyle’s law? … Boyle’s law states that at constant temperature for a fixed mass, the absolute pressure and the volume of a gas are inversely proportional. The law can also be stated in a slightly different manner, that the product of absolute pressure and volume is always constant.
How do you calculate Boyle’s Law?
Then, the equation of Boyle’s law states that: p₂ = p₁ * V₁ / V₂ or p₂ / p₁ = V₁ / V₂ . As we can see, the ratio of the final and initial pressure is the inverse of the ratio for volumes.
What does p1v1 p2v2 mean?
… The relationship for Boyle’s Law can be expressed as follows: P1V1 = P2V2, where P1 and V1 are the initial pressure and volume values , and P2 and V2 are the values of the pressure and volume of the gas after change.
Where is Boyle’s law used?
Since the boiling point is dependent on pressure, you can use Boyle’s law and a syringe to make water boil at room temperature. Deep-sea fish die when they’re brought from the depths to the surface. The pressure decreases dramatically as they are raised, increasing the volume of gases in their blood and swim bladder.
What are the 3 gas laws?
The gas laws consist of three primary laws: Charles’ Law, Boyle’s Law and Avogadro’s Law (all of which will later combine into the General Gas Equation and Ideal Gas Law).
Does Boyle’s law have to be in ATM?
There are also two volume variables; they also must have the same unit. … Its pressure changes to 1.93 atm.
Why is Boyle’s law true?
Boyle’s Law holds true only if the number of molecules (n) and the temperature (T) are both constant. Boyle’s Law is used to predict the result of introducing a change in volume and pressure only, and only to the initial state of a fixed quantity of gas.
What is K in Boyle’s law?
Simply put, Boyle’s states that for a gas at constant temperature, pressure multiplied by volume is a constant value. The equation for this is PV = k, where k is a constant. At a constant temperature, if you increase the pressure of a gas, its volume decreases. … Boyle’s law is a form of the Ideal Gas Law.