Why are solvent pairs used in recrystallization? In recrystallization, two solvents are used to either induce crystallization or improve crystal growth. The recrystallization can be done with a second solvent, usually with a lower b.p. but the compound of interest is very soluble (e.g. diethyl ether).
Why are two solvents used in recrystallization?
Two solvents are used in recrystallization in order to either induce crystallization or to improve the growth of crystals. For example say a compound has poor solubility in petroleum spirit (60–80) regardless of the temperature. A second solvent, typically with a lower b.p.,...
What is the principle of recrystallization?
Recrystallization’s principle is that the amount of solute dissolved by a solvent rises with temperature. A solution is created in recrystallization when a solute is dissolved in a solvent at or near the boiling point. Recrystallization only works when the correct solvent is used.
How do you recrystallize a mixture?
Recrystallisation from mixed solvents is carried out near the boiling point of the solvent. The compound is dissolved in the solvent in which it is very soluble, and the hot solvent, in which the substance is only sparingly soluble, is added cautiously until a slight turbidity is produced.
Can a recrystallization yield be 100%?
Necessary sources of mass loss: The yield for a recrystallization can never be 100%. Why not? Because while the chilled solvent is saturated and should release some crystals, at least some of your desired material will remain dissolved in the cold solvent and will be lost when the crystals and solvent are separated.
Why we use a solvent pair in recrystallization?
It is necessary to use a solvent pair recrystallization as one can dissolve the crystals in the better solvent and then the hot solution is added with the poorer solvent until the solution becomes completely cloudy. This simply means that the solution has been saturated with the solute.
Why do the two solvent you use in mixed solvent recrystallization have to be miscible?
The two solvents must be miscible in one another so that their solubility with one another does not limit the proportions used. Table 3.2 shows a list of common mixed solvents used in crystallization.
Why are solvent pairs such as water and ethanol sometimes employed for recrystallization?
Ethanol/water combinations are commonly used because ethanol has good dissolving ability for many organics, but is also infinitely co-soluble with water. Addition of water can rapidly and dramatically reduce the solubility of many organics and thus induce crystallization.
Why are solvent pairs used in some cases instead of a single solvent?
When no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. A pair of solvents is chosen: one in which the compound is soluble (called the "soluble solvent"), and one in which the compound is insoluble (called the "insoluble solvent").
How do you choose a solvent pair for recrystallization?
The solvent should be nontoxic, noncarcinogenic, and nonflammable, non-volatile. The solvent should boil in the range 50–120°C. Impurities should either be insoluble in the hot solvent or soluble in the cold solvent. The solvent must not react with the compound.
How do you choose a solvent for recrystallization?
An ideal crystallization solvent should be unreactive, inexpensive, and have low toxicity. It is also important that the solvent have a relatively low boiling point (b.p. often <100oC as it's best if the solvent readily evaporates from the solid once recovered.
What makes a solvent pair too good?
A solvent which is "too good" will not allow recovery of much of the compound. On the other hand, if the solvent is "too poor," an excessively large volume of solvent would be needed. A solvent should be fairly volatile, because after the compound is collected, it must be freed of adsorbed solvent.
Why is water used as a solvent in recrystallization of aspirin?
Because aspirin is less soluble in cold water. In fact, aspirin is not very soluble in water at all, which is why you are supposed to take it with lots of water.
What advantages and disadvantages does water have as a recrystallization solvent?
Water. Answer: Advantage is its low cost and low toxicity; disadvantage is the difficulty of removing it from products due to low volatility.
Why do impurities remain in the solvent during a recrystallization?
Because the soluble impurities are present in smaller amounts, the solution never becomes saturated with the impurities, so the impurities remain in solution even after the solution has cooled. Removing the solution from the crystals thus removes the solvent and the soluble impurities from the desired crystals.
How do you choose the appropriate solvent in recrystallization method to isolate and purify certain solid drug preparations?
The criteria used to choose an appropriate recrystallization solvent includes: a.) finding a solvent with a high temperature coefficient. The solvent must not dissolve the compound at low temperatures (that includes room temperature), but must dissolve the compound at high temperatures.
Why is cold solvent used to wash crystals?
Washing the crystals Once the suction filtration process is complete the collected crystals should be washed with a little more ice–cold solvent to remove final soluble impurities which would otherwise be left on the surface of the crystals.
When choosing a recrystallization solvent or binary solvent system what are the properties one should consider?
An ideal crystallization solvent should be unreactive, inexpensive, and have low toxicity. It is also important that the solvent have a relatively low boiling point (b.p. often <100oC as it's best if the solvent readily evaporates from the solid once recovered.
How do you recrystallize two solvents?
Overview: For a two-solvent recrystallization, you should have one solvent (solvent #1) in which your desired compound is soluble at the boiling point. The second solvent (solvent #2) should induce crystallization when added to a saturated solution of your compound in the primary solvent.
Which solvents are generally used as a solvent for crystallization?
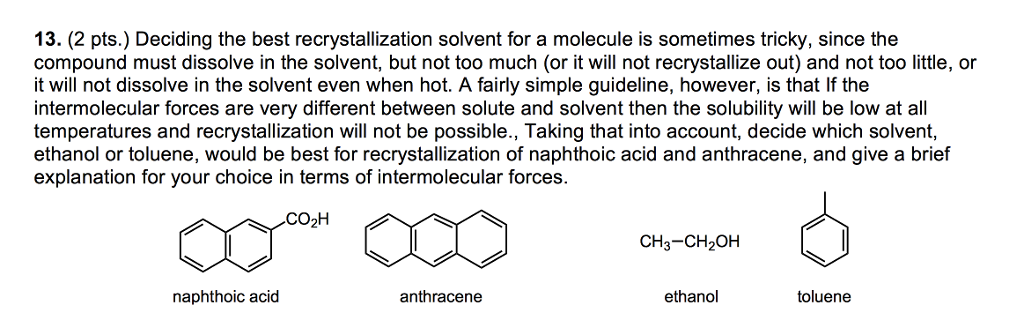
Much of crystallization uses common laboratory solvents, such as water, alcohols, acetone, ethyl acetate, cyclohexane, and toluene; it is also wise to recall the “like dissolves like” dictum.
What are the characteristics of a good recrystallization solvent?
A good recrystallization solvent should (1) dissolve a moderate quantity of the substance being purified at an elevated temperature, but only a small quantity at low temperatures, (2) not react with the substance being purified, (3) dissolve impurities readily at a low temperature or not dissolve them at all, and (4) ...
Why do scientists use recrystallization?
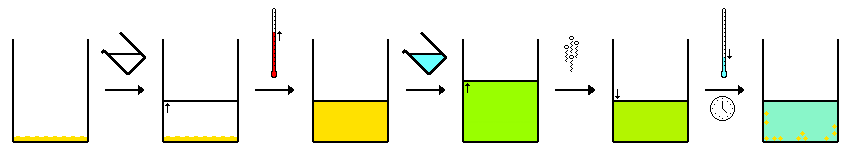
Scientists use recrystallization to purify solids, typically products, from different chemical reactions. The process involves dissolving a solid into solution, then allowing the dissolved substance to gradually crystallize. This produces compounds high in purity, a quality which can be seen by the presence of uniform crystals.
Why do scientists use multiple solvents?
Scientists typically employ multiple solvents when no singular solvent meets all the criteria for that given recrystallization procedure. Each solvent pair contains a “soluble solvent,” one in which the compound is soluble, and an “insoluble solvent,” one in which the compound is insoluble. The soluble solvent and insoluble solvent must be miscible, so that the proportions used do not limit their solubility in solution.
What is the purification of a compound?
The definition of recrystallization is a technique for the purification of compounds in which a compound is dissolved in a solvent and slowly cooled to form crystals, which are a purer form of the compound.
What happens to the crystals in a solution as the temperature decreases?
As the temperature decreases, so does the solubility of the compound in solution. Soon, the solution becomes supersaturated, prompting crystal formation. As discussed, these crystals then precipitate out of the solution at the cooler recrystallization temperature.
How to accelerate crystallization?
To expedite crystallization and recrystallization processes, scientists can employ a technique called seeding. Seeding involves first dipping a small crystal, or seed, of solute into the saturated solution. Following this step, larger crystals can form and subsequently grow on this seed crystal.
What is the application of recristallization?
Recrystallization has applications that extend into the industrial, medical, and pharmaceutical industries. Techniques such as texture control, drug development, and treatment purification all involve the procedure.
How much of a compound is needed for recrystallization?
But this substance must contain approximately 80% of your desired compound; in other words, it should be mostly pure. Otherwise, the recrystallization will not proceed smoothly.
Can a mixture of solvents be used for recrystallization?
If the substance is found to be far too soluble in one solvent and much too insoluble in another solvent to allow of satisfactory recrystallisation, mixed solvents or solvent pairs may frequently be used with excellent results. The two solvents must, of course, be completely miscible. Recrystallisation from mixed solvents is carried out near ...
Can a mixture of two solvents be miscible?
The two solvents must, of course, be completely miscible. Recrystallisation from mixed solvents is carried out near the boiling point of the solvent. The compound is dissolved in the solvent in which it is very soluble, and the hot solvent, in which the substance is only sparingly soluble, is added cautiously until a slight turbidity is produced.
Can thiirane be crystallized?
The thiirane usually can be crystallized from an appropriate solvent pair. Chromatography over alumina has been used for the purification of episulfides. [Pg.45] When inserted into an oil bath at 200°, the compound undergoes an immediate change in crystal structure and melts at 232-234°.
Is it dangerous to add hot solvents to one another?
The addition of hot solvents to one another can be tricky. It can be extremely dangerous if the boiling points of the solvents are very different. For the water-methanol mixed solvent, if 95 °C water hits hot methanol (B.P. 65.0 °C), watch out ...

CORE Concepts
Topics Covered in Other Articles
What Is Recrystallization
- The definition of recrystallizationis a technique for the purification of compounds in which a compound is dissolved in a solvent and slowly cooled to form crystals, which are a purer form of the compound. Scientists use recrystallization to purify solids, typically products, from different chemical reactions. The process involves dissolving a soli...
Recrystallization vs Crystallization
- Although the procedures of crystallization and recrystallization display similarities, their respective definitions diverge. Firstly, crystallization is a separation technique. It involves precipitating crystals from solution through transitions in the solute’s solubility conditions. The resulting crystals can be readily distinguished and subsequently filtered out from the solution. S…
Types of Recrystallization
- 1. Single Solvent Recrystallization
This type of recrystallization is the most basic and, as a result, the most frequently employed. The process involves dissolving a compound, “A,” and impurity, “B,” in a heated solvent system. As this solution cools back to room temperature, the solubility of the compounds in the solution drops, … - 2. Multi-Solvent Recrystallization
This method of recrystallization closely resembles the first in terms of the procedure involved. However, multi-solvent recrystallization requires two, and sometimes more, solvents. The compound, “A,” and impurity, “B,” dissolve in the first solvent. Then, the addition of a second solv…
How to Perform A Simple Recrystallization Procedure
- Below is a stepwise guidefor completing a basic recrystallization procedure. 1. First, weigh the impure solvent and record that value. Then add the impure compound to a solvent system. 2. Heat this solvent system to your target temperature, or its boiling point. Be sure to raise the temperature in gradual increments to ensure that the rest of the process proceeds smoothly. 3. …
Limitations of Recrystallization
- Although quite useful, recrystallization possesses some limitations and ramifications. Firstly, the compound in question must occupy a solid state at standard conditions. This means that substances including oils, greases, and waxes cannot be crystalized or recrystallized under standard conditions. The purity of your crude material also comes into play. The success of recr…
Applications of Recrystallization to Today’S World
- Recrystallization has applications that extend into the industrial, medical, and pharmaceutical industries. Techniques such as texture control, drug development, and treatment purification all involve the procedure. But the pharmaceutical industry actually makes the most use of recrystallization procedures. Purification and separation processes are key to the isolation of dif…