- Two hydrogen atoms and one oxygen atoms are near each other.
- When two hydrogen atoms come close enough to an oxygen atoms, their electrons are attracted to the proton of the other atom.
- Because there is both a strong enough attraction between atoms and room for electrons in the outer energy levels of the atoms, they share electrons. ...
What kind of covalent bond has unequal sharing of electrons?
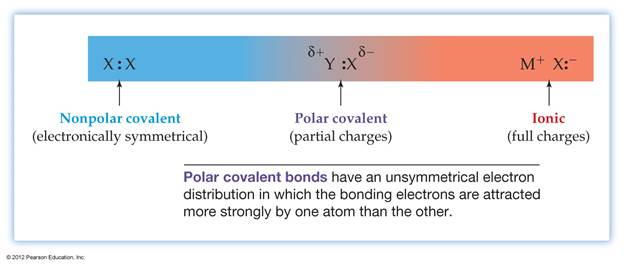
The unequal sharing of electrons in a covalent bond is called polar covalent bond. As for example- H and Cl both atoms share one electron to each other to form H-Cl bond. In H-Cl bond, Cl is more electronegative atom. For this,the sharing electrons are strongly attracted by chlorine atom.
Which covalent bond is the strongest and why?
The Covalent Bond formed in DIAMOND is the strongest as far as I know. The C-C bond is 100% covalent in nature and is the strongest bond. The hardness and strength of the diamond molecule is due to the Molecular network (as well as in Quartz ). A Diamond molecule has a continuous C-C bonds and forms a giant molecule.
Why is the covalent bond called a true chemical bond?
covalent bonding. also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. polar covalent bond.
Why is hydrogen bond a weaker bond than covalent bond?
Hydrogen bond is formed by the weak electrostatic attraction forces between the positive pole of one molecule and the negative pole of another molecule generally of the same substance. So it is more stronger than covalent bond. Consequently, the hydrogen bond is very much weaker than covalent bond and ionic bond. Click to see full answer.

Why do atoms share electrons in covalent bonds quizlet?
Why do atoms share electrons in covalent bonds? to attain a stable noble-gas electron configuration.
Why do covalent bonds share electrons instead of transfer electrons?
A covalent bond is formed when pairs of electrons are shared between atoms. The reason why electrons are shared is related to the overall stability of the atoms. Rather than transferring electrons in a covalent bond, atoms in non-metals share pairs of electrons in order to achieve stability.
Why do atoms share electrons in covalent bonds Think of electronegativity?
Covalent Bonding If atoms have similar electronegativities (the same affinity for electrons), covalent bonds are most likely to occur. Because both atoms have the same affinity for electrons and neither has a tendency to donate them, they share electrons in order to achieve octet configuration and become more stable.
Why do atoms gain or share electrons?
Many atoms become stable when their valence shell is filled with electrons or when they satisfy the octet rule (by having eight valence electrons). If atoms don't have this arrangement, they'll “want” to reach it by gaining, losing, or sharing electrons via bonds.
What does it mean for electrons to be shared?
Electron 'sharing' occurs when the electrons in the outermost electron shell, or valence shell electrons, from one atom can be used to complete the outermost electron shell of another atom without being permanently transferred, as occurs in the formation of an ion.
Is covalent bond equal sharing?
Covalent bonds involve the equal sharing of an electron pair by two atoms.
Why are electrons in the covalent bonds of water shared unequally?
Covalent bonds occur when two atoms—in this case oxygen and hydrogen—share electrons with each other. Because oxygen and hydrogen attract the shared electrons unequally, each end of the V-shaped H2O molecule adopts a slightly different charge.
Why do ionic bonds transfer electrons?
In ionic bonding, electrons are completely transferred from one atom to another. In the process of either losing or gaining negatively charged electrons, the reacting atoms form ions. The oppositely charged ions are attracted to each other by electrostatic forces, which are the basis of the ionic bond.
Why do atoms form ionic and covalent bonds?
An ionic bond, where one atom essentially donates an electron to another, forms when one atom becomes stable by losing its outer electrons and the other atoms become stable (usually by filling its valence shell) by gaining the electrons. Covalent bonds form when sharing atoms results in the highest stability.
Why do atoms share electrons Quizizz?
Q. Why do atoms share electrons? To attain the electron configuration of a noble gas.
Do covalent bonds share or transfer electrons?
A covalent bond consists of the mutual sharing of one or more pairs of electrons between two atoms. These electrons are simultaneously attracted by the two atomic nuclei. A covalent bond forms when the difference between the electronegativities of two atoms is too small for an electron transfer to occur to form ions.
Are electrons fully transferred between atoms in A covalent bond?
Bonding between non-metals consists of two electrons shared between two atoms. In covalent bonding, the two electrons shared by the atoms are attracted to the nucleus of both atoms. Neither atom completely loses or gains electrons as in ionic bonding.
What type of bond is formed by the transfer of electrons?
ionic bondAn ionic bond is formed by the complete transfer of some electrons from one atom to another. The atom losing one or more electrons becomes a cation—a positively charged ion. The atom gaining one or more electron becomes an anion—a negatively charged ion.
Is ionic sharing or transferring electrons?
An ionic bond is actually the extreme case of a polar covalent bond, the latter resulting from unequal sharing of electrons rather than complete electron transfer.