
Fibrous proteins have a limited number of amino acids with the sequence usually being highly repetitive The highly repetitive sequence creates very organised structures that are strong and this along with their insolubility property, makes fibrous proteins very suitable for structural roles Examples of fibrous proteins:
Full Answer
Why study the fine structure of fibrous proteins?
Since fibrous proteins generally have periodical amino acid sequences and higher-order structure, the clarification of their fine structure in the solid state becomes very important not only when discussing the physical and chemical properties, but when obtaining information about the molecular design of synthetic polypeptides.
What is the primary structure of a protein?
The amino acid sequence is known as the primary structure of the protein. Stretches of polypeptide chain that form α helices and β sheets constitute the protein’s secondary structure.
What is an example of fibrous protein?
Fibrous proteins often play a structural role in nature. For example, α-keratin is composed of α-helical segments of polypeptides and is the predominant constituent of claws, fingernails, hair, and horn in mammals.
What determines the shape of a protein?
As a result of all of these interactions, each type of protein has a particular three-dimensional structure, which is determined by the order of the amino acids in its chain. The final folded structure, or conformation, adopted by any polypeptide chain is generally the one in which the free energy is minimized.
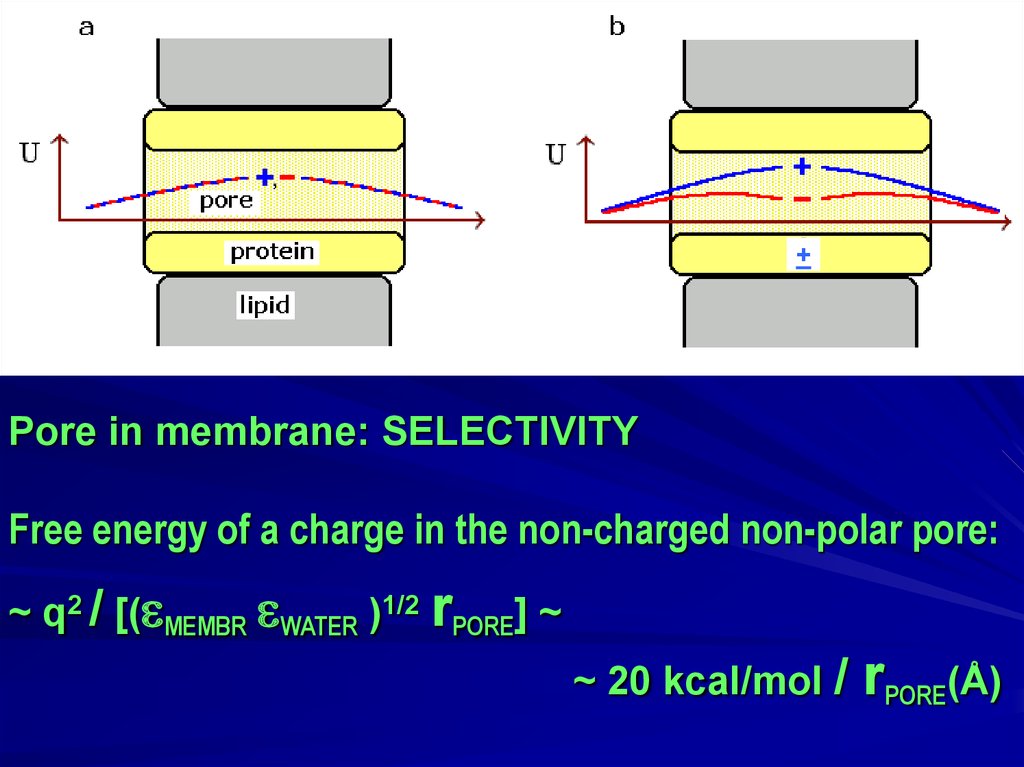
Do fibrous proteins have repetitive amino acid sequences?
The fibrous proteins have some commonality of amino acid sequence. Each possesses an abundance of repeating sequences of amino acids with small, non-reactive side groups. Many contain short repeats of sequences, often with glycine.
Why do fibrous proteins have a structural function?
Fibrous proteins are mainly used as structural proteins due to their insolubility and strength, meaning they are formidable against damage and create water-tight seals, as well as their long polypeptide chains being ideal for large biological structures.
How are fibrous proteins held together?
In fibrous proteins, polypeptide chains are held together by hydrogen and disulphide bond, in parallel manner. Due to which fibre-like structure is obtained. Such proteins are generally known as fibrous proteins. These proteins are generally insoluble in water.
What are the characteristic of a fibrous protein?
Fibrous proteins are characterized by elongated shape of molecules. Long stretches of keratin, myosin, and fibrinogen contain coiled coil structure - a pair of a-helices winded around each other along their entire length. Collagen three strands form a triple helix.
Why are fibrous proteins more stable than globular?
Fibrous proteins generally have no tertiary structure to denature which makes them quite stable as far as structure goes. They also have many nonpolar residues on their surface which makes them less soluble in water than many globular proteins.
How does fibrous proteins differ from globular protein?
Fibrous proteins are structural in nature, which means they help maintain cell shape by providing a scaffolding or a framework. On the other hand, globular proteins are functional, which means they carry out a specific biological function in the body.
Which type of bond is present in fibrous protein?
Solution : Hydrogen bonds and disulphide bonds.
Why are fibrous proteins strong?
Fibrous proteins contain polypeptide chains organized approximately in parallel along a single axis, producing long fibers or large sheets. Such proteins tend to be mechanically strong and resistant to solubilization in water. Fibrous proteins often play a structural role in nature.
Why are fibrous proteins insoluble?
Explanation: Fibrous proteins do not dissolve in water due to the difference in polarity. According to chemical laws, "like dissolves like". Since water is polar, and the surface of fibrous proteins is covered in non-polar amino acids, it does not dissolve into the aqueous solution.
Which of the following is a general function for a fibrous proteins?
Fibrous proteins consist of elongated polypeptide chains that run parallel to one another and are stabilized by cross-linkages. In humans, their main role is to provide structure and support and aid in biomechanics.
How are fibrous proteins formed?
Fibrous proteins are long chains. They are made up of repeated amino acid sequences that form long polypeptide chains. These chains twist together to form fibrous proteins.
What are the basic features of fibrous proteins like a keratin and collagen?
Fibrous proteins are made up of polypeptide chains that are elongated and fibrous in nature or have a sheet like structure. These fibers and sheets are mechanically strong and are water insoluble. They are often structural proteins that provide strenth and protection to cells and tissue.
What structure is fibrous protein?
Fibrous proteins contain polypeptide chains organized approximately in parallel along a single axis, producing long fibers or large sheets. Such proteins tend to be mechanically strong and resistant to solubilization in water. Fibrous proteins often play a structural role in nature.
Which of the following is the most common function for fibrous proteins?
These fibrous proteins are insoluble in water and usually serve structural, connective, and protective functions. Examples of fibrous proteins are keratins, collagens, myosins, and elastins.
Do fibrous proteins function as enzymes?
Globular proteins have multiple functions as they are used to form enzymes, cellular messengers, amino acids but fibrous proteins act only as structural proteins.
What are the major structural features of proteins in silk and keratin?
Silk or silk fibers are often considered fibroin protein block copolymer structures because the primary structure of the fibers is composed of repetitive block of glycine and alanine amino acids. This primary structure forms a secondary structure of complex chains of β-keratin, antiparallel pleated crystal blocks (Fig.
What are the two fold patterns of proteins?
Both patterns were discovered about 50 years ago from studies of hair and silk. The first folding pattern to be discovered, called the α helix, was found in the protein α- keratin, which is abundant in skin and its derivatives—such as hair, nails, and horns. Within a year of the discovery of the α helix, a second folded structure, called a β sheet, was found in the protein fibroin, the major constituent of silk. These two patterns are particularly common because they result from hydrogen-bonding between the N–H and C=O groups in the polypeptide backbone, without involving the side chains of the amino acids. Thus, they can be formed by many different amino acid sequences. In each case, the protein chain adopts a regular, repeating conformation. These two conformations, as well as the abbreviations that are used to denote them in ribbon models of proteins, are shown in Figure 3-9 .
What type of bonds help proteins fold?
Three types of noncovalent bonds that help proteins fold. Although a single one of these bonds is quite weak, many of them often form together to create a strong bonding arrangement, as in the example shown. As in the previous figure, R is used as a general (more...)
What is the refolding of a denatured protein?
The refolding of a denatured protein. (A) This experiment demonstrates that the conformation of a protein is determined solely by its amino acid sequence. (B) The structure of urea. Urea is very soluble in water and unfolds proteins at high concentrations, (more...)
How do amino acids fold into compact conformations?
The polar amino acid side chains tend to gather on the outside of the protein, where they can interact with water; the nonpolar amino acid side chains are buried on the inside to form a tightly packed hydrophobic (more...)
What is a protein made of?
A protein molecule is made from a long chain of these amino acids, each linked to its neighbor through a covalent peptide bond ( Figure 3-1 ). Proteins are therefore also known as polypeptides. Each type of protein has a unique sequence of amino acids, exactly the same from one molecule to the next.
What are the structural components of a protein?
The structural components of a protein. A protein consists of a polypeptide backbone with attached side chains. Each type of protein differs in its sequence and number of amino acids; therefore, it is the sequence of the chemically different side chains (more...)
How many entries are there in the protein database?
The present database of known protein sequences contains more than 500,000 entries, and it is growing very rapidly as more and more genomes are sequenced—revealing huge numbers of new genes that encode proteins. Powerful computer search programs are available that allow one to compare each newly discovered protein with this entire database, looking for possible relatives. Homologous proteins are defined as those whose genes have evolved from a common ancestral gene, and these are identified by the discovery of statistically significant similarities in amino acid sequences.
