
A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. Synthesis Reaction Combination Reaction In a synthesis reaction two or more substances combine to form a new compound. Single replacement reactions occur if the lone element can replace a similar element in a compound.
What are some real-life examples of single replacement reactions?
Here are some examples of single replacement reactions:
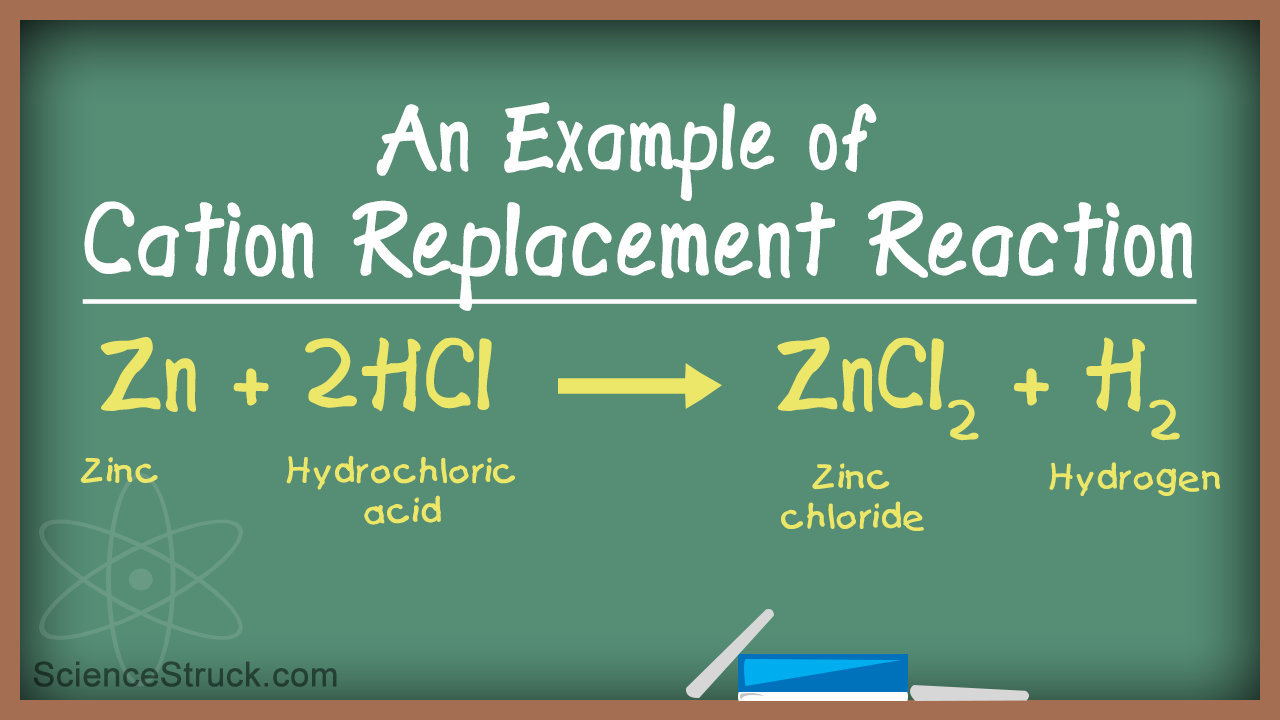
- zinc + hydrochloric acid ---> zinc chloride and hydrogen gas
- zinc + silver nitrate ---> zinc nitrate and silver metal
- calcium + water ---> calcium hydroxide and dihydrogen
- iron + copper nitrate ---> iron nitrate and copper metal
- bromine + potassium iodide ---> potassium bromine and iodine
What is a real life example of double replacement reaction?
An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. Both silver nitrate and sodium chloride are ionic compounds. Both reactants dissolve into their ions in aqueous solution. What is another name for double-replacement reaction?
What are single replacement and double replacement reactions?
Key Takeaways
- A single-replacement reaction replaces one element for another in a compound.
- The periodic table or an activity series can help predict whether single-replacement reactions occur.
- A double-replacement reaction exchanges the cations (or the anions) of two ionic compounds.
What occurs during a single replacement reaction?
A single-replacement reaction occurs when a single element is replaced by another element that is part of a compound. A double-replacement reaction occurs when two elements or ions of two different compounds are switch or replaced with each other.
Why do some single replacement reactions not occur?
Because iodine is below chlorine on the periodic table, a single-replacement reaction will not occur. Because fluorine is above bromine on the periodic table, a single-replacement reaction will occur, and the products of the reaction will be CaF2 and Br2.
Will single replacement reactions always occur?
To determine whether a given single replacement will occur, you must use an “Activity Series” table. If the metal or the halogen is above the element it will replace based on the activity series, a single displacement reaction will occur.
What are the rules of a single replacement reaction?
In a single replacement reaction, a single element replaces an atom in a compound producing a new compound and a pure element. Like double replacement reactions, metals always replace metals and nonmetals always replace nonmetals in a compound.
How do you know a single displacement reaction has occurred?
Some of the observable signs that a chemical reaction has occurred include the following:A metallic deposit appears.Bubbles appear.A temperature change occurs.A color change occurs.A precipitate (cloudy, tiny particles) appears.
How do you determine if a reaction will occur?
There are five (easy) ways to detect a reaction:Color Change.Precipitate Formation (solid formation falling out of solution)Gas Formation (bubbles and odor)Temperature Change.pH Change.
Why do reactions not occur?
When molecules collide, the kinetic energy of the molecules can be used to stretch, bend, and ultimately break bonds, leading to chemical reactions. If molecules move too slowly with little kinetic energy, or collide with improper orientation, they do not react and simply bounce off each other.
How do you predict whether or not a double displacement reaction will occur?
To judge whether double-replacement reactions will occur, we need to know what kinds of ionic compounds form precipitates. For this, we use solubility rules, which are general statements that predict which ionic compounds dissolve (are soluble) and which do not (are not soluble or insoluble).
How do you know if there is no reaction in a chemical equation?
Write another chemical equation you want to analyze; for example, the combination of sodium chloride (NaCl) and calcium nitrate (Ca[NO3]2): "NaCl(aq) + Ca(NO3)2(aq) --> NR." Remember that "NR" means "no reaction." Note that there are no chemical terms to the right of the arrow.
How many types of replacement reactions are there?
There are two types of replacement reactions, observe the differences:
What is single displacement reaction?
In a single-displacement reaction, one element displaces another element in a compound. A and B must be either different metals (including H) or different halogens. Chemists have devised an Activity Series. It lists the metals and nonmetals in decreasing order of chemical activity.
How to determine if a single replacement will occur?
To determine whether a given single replacement will occur, you must use an “Activity Series” table. If the metal or the halogen is above the element it will replace based on the activity series, a single displacement reaction will occur. Examples:
What is a decomposition reaction?
A decomposition reaction is one where a compound is broken down into its constituent chemical species:
What Is a Single-Displacement Reaction?
We replace our possessions because the replacements will better suit our purposes, or because they will serve us better in the future. This is comparable to single-displacement reactions in chemistry.
Why do we replace something?
Just like we said earlier, we usually replace something if the replacement is better or will suit our purposes better. The replacement is usually similar to the original object. In the same way, for a single-displacement reaction, an element can only be replaced if the element taking its place is more reactive.
Why does Cu replace Ag?
For example, in this reaction, Cu replaces Ag because Cu is more reactive than Ag. We can confirm that by looking at the activity series of metals.
What is the subscript of Br when Cl replaces Br?
We need to take note that when Cl replaces Br, Br has a subscript of 2 in the products side. This is because Cl and Br are both in the same group in the periodic table, so we know they behave similarly.
Why put a coefficient of 2 in front of HCl?
In this case, we put a coefficient of 2 in front of HCl to balance the H and Cl atoms on both sides.
Which element easily loses electrons to form a cation?
8. Potassium (K), easily loses electrons to form a cation _____
Can Zn replace H?
This tells us that Zn can replace H, so this reaction will occur. Step two: Determine the products. When H is replaced by Zn, H will naturally occur as H2, so on the products side, one of the products is H2. Zinc, a metal, will combine with Cl, a nonmetal.
What is single replacement reaction?
A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. To determine whether a given single replacement will occur, you must use an “Activity Series” table.
How to determine if a single replacement will occur?
To determine whether a given single replacement will occur, you must use an “Activity Series” table. If the metal or the halogen is above the element it will replace based on the activity series, a single displacement reaction will occur. Examples:
